A solution is formed by mixing 50.0 mL of 10.0 M NaX with 50.0 mL of 2.0
Question:
A solution is formed by mixing 50.0 mL of 10.0 M NaX with 50.0 mL of 2.0 × 10-3 M CuNO3. Assume that Cu+ forms complex ions with X- as follows:
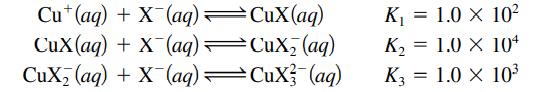
with an overall reaction
![]()
Calculate the following concentrations at equilibrium.
a. CuX32-
b. CuX2-
c. Cu+
Transcribed Image Text:
Cu (aq) + CuX(aq) + X(aq) CuX₂ (aq) + X(aq) X(aq)=CuX(aq) CuX₂ (aq) CuX (aq) K₁ = 1.0 X 10² K₂ = 1.0 X 104 K3 = 1.0 × 10³ X
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
Solution a C...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
From the following cash flow diagram, find the value of C that will establish economic equivalence between the deposit series and the withdrawal series at an interest rate of 9% compounded annually....
-
Pin fins are to be specified for use in an industrial cooling application. The fins will be subjected to a gas in cross flow at V = 10 m/s. The cylindrical fin has a diameter of D = 15 mm, and the...
-
An ideal dilute solution is formed by dissolving the solute A in the solvent B. Write expressions equivalent to Equations (9.9) through (9.13) for this case.
-
Consider the deletion of record 5 from the file as shown below compare the relative merits of the following techniques for implementing the deletion: a. Move record 6 to the space occupied by record...
-
How does a chattel mortgage differ from a conditional sales contract when it comes to financing equipment?
-
Benzene and toluene form nearly ideal solutions. The boiling point of pure benzene is 80.1*C, Calculate the chemical potential of benzene relative to that of pure benzene when xbmzenc = 0.30 at its...
-
The number of books per shelf in a library (a) find the mean, variance, and standard deviation of the probability distribution, (b) interpret the results. Books 0 1 2 3 4 5 Probability 0.002 0.018...
-
Refer to Cornerstone Exercise 7.3. Now assume that Valron Company uses the sequential method to allocate support department costs. The support departments are ranked in order of highest cost to...
-
Evaluate the implications of fluid-structure interactions (FSI) in engineering design, considering the effects of fluid-induced forces on structural integrity, the occurrence of phenomena such as...
-
Each of the flowchart segments in Figure 3-24 is unstructured. Redraw each segment so that it does the same processes under the same conditions, but is structured. a. D Yes NO Yes B? E? No F H C. Yes...
-
A solution contains 1.0 10 -5 M Na 3 PO 4 . What concentrations of AgNO 3 will cause precipitation of solid Ag 3 PO 4 (K sp = 1.8 10 -18 )?
-
If 10.0 mL of 2.0 10 3 M Cr(NO 3 ) 3 is added to 10.0 mL of a pH = 10.0 NaOH solution, will a precipitate form?
-
In Exercises find the positive values of p for which the series converges. n(1 + n?)P n=1
-
Prepare the adjusting journal entries that were recorded on December 31, 2024. The general ledger of the Karlin Company, a consulting company, at January 1, 2024, contained the following account...
-
Selected comparative financial statements of Korbin Company follow. KORBIN COMPANY Comparative Income Statements For Years Ended December 31 Sales 2021 $ 559,409 2020 2019 $ 428,553 $ 297,400 Cost of...
-
Sun Corporation is a manufacturer of a single product. The following information relates to its production levels and costs for 2009: Number of units produced = 7.5 million Cost of raw materials =...
-
Let R stand for radiation exposure, t stand for length of time of exposure, r stand for distance, and k be the constant of variation. Write an equation of joint variation representing the...
-
Selected data for the consulting department of Austin Consulting, Inc., follow: Estimated consulting overhead cost for the year $ 3 6 0 , 0 0 0 Estimated direct labor cost for the year ( @ $ 9 / hr ....
-
For a fixed type 1 error rate, how can a test minimize the probability of a type 2 error?
-
Provide a few individual examples who revealed what aspects of emotional intelligence?
-
Which of the following compounds are chiral? Draw them, and label the chirality centers. (a) 2, 4-Dimethylheptane (b) 5-Ethyl-3, 3-dimethylheptane (c) cis-l, 4-Dichlorocyclohexane (d) 4,...
-
A Draw chiral molecule that meet the following descriptions: (a) A chloro alkane, C5H11C1 (b) An alcohol, C6H14O (c) An alkene, C6H12 (d) An alkane, C8H18
-
A Eight alcohols have the formula C5H120. Draw them. Which are chiral?
-
The blue samurai, a japanese restraurant, has an asset turnover of 3.5 the total assets were 95,000 what are net sales for the blue samurai?
-
Gatekeeper Manufacturing reported 50,000 physical units that were 100% complete for direct materials during the period. In addition, the 50,000 physical units were 100% for conversion costs. In terms...
-
What red flag was overlooked on the Montague Fellowship Expense Report?

Study smarter with the SolutionInn App


