Consider two reaction vessels, one containing A and the other containing B, with equal concentrations at t
Question:
Consider two reaction vessels, one containing A and the other containing B, with equal concentrations at t = 0. If both substances decompose by first-order kinetics, where
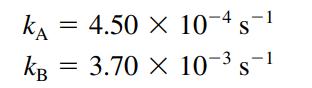
how much time must pass to reach a condition such that [A] = 4.00[B]?
Transcribed Image Text:
KA KB = 4.50 x 10-4 S-1 3.70 X 10-3 S-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (10 reviews)
Answer It must pass 71 min Explanation For first order kinetics the concentration of ...View the full answer

Answered By

Marvine Ekina
Marvine Ekina
Dedicated and experienced Academic Tutor with a proven track record for helping students to improve their academic performance. Adept at evaluating students and creating learning plans based on their strengths and weaknesses. Bringing forth a devotion to education and helping others to achieve their academic and life goals.
PERSONAL INFORMATION
Address: , ,
Nationality:
Driving License:
Hobbies: reading
SKILLS
????? Problem Solving Skills
????? Predictive Modeling
????? Customer Service Skills
????? Creative Problem Solving Skills
????? Strong Analytical Skills
????? Project Management Skills
????? Multitasking Skills
????? Leadership Skills
????? Curriculum Development
????? Excellent Communication Skills
????? SAT Prep
????? Knowledge of Educational Philosophies
????? Informal and Formal Assessments
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Consider two reaction vessels, one containing A and the other containing B, with equal concentrations at t = 0. If both substances decompose by first-order kinectics, where kA = 4.50 10-4 s-1 kB =...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Consider studying whether drug A and drug B are equally effective in lowering blood pressure. For each of (a), (b), and (c) answer two questions (you do not need to explain your answers): (I) What...
-
Ashby and Curtis, married professionals, have a 2-year-old son, Jason. Curtis works full-time as an electrical engineer, but Ashby has not worked outside the home since Jason was born. As Jason is...
-
What is the decision facing American Express?
-
Thickness measurements of a coating process are made to the nearest hundredth of a millimeter. The thickness measurements are uniformly distributed with values 0.15, 0.16, 0.17, 0.18, and 0.19....
-
A Pelton wheel is supplied with water from a lake at an elevation \(H\) above the turbine. The penstock that supplies the water to the wheel is of length \(\ell\), diameter \(D\), and friction factor...
-
Gibson Agency Case: 1. Calculate and present the budgeted profit for each of Gibson's clients for each of the years 2016 through 2019, using the current costing system (i.e., the one described in the...
-
An ASTM A572 Grade 50 steel bar is attached to a rigid wall at one end and a spring at the other end, with the other end of the spring being attached to a rigid wall, as shown below. The bar is 20 cm...
-
Effective financial statement analysis requires an understanding of a firms economic characteristics. The relations between various financial statement items provide evidence of many of these...
-
Cobra venom helps the snake secure food by binding to acetylcholine receptors on the diaphragm of a bite victim, leading to the loss of function of the diaphragm muscle tissue and eventually death....
-
Theophylline is a pharmaceutical drug that is sometimes used to help with lung function. You observe a case where the initial lab results indicate that the concentration of theophylline in a patients...
-
Materials used by the Truck Division of Goldman Motors are currently purchased from outside suppliers at a cost of $310 per unit. However, the same materials are available from the Components...
-
You are currently in school to get your associate degree for health services Administration the portfolio project was based on this : You have volunteered to organize a fundraiser for your child's...
-
Explain the differences between defined contribution and defined benefits plans, also include their benefits and shortfalls
-
What policies, is any, should employers develop concerning the use of social media for various purposes, including employee recruitment and selection
-
In late adulthood, there are changes that occur physically and cognitively which are at times difficult for the individual to embrace. Please share at least2physical (one must relate to sex or...
-
Why is it important to provide employees feedback on performance? What are the ramifications if we don't
-
Convert 562 mmHg to atm.
-
Give an example of transitory income. What effect does this income have on the marginal propensity to consume?
-
A student wishes to record the UV spectrum of trans-stilbene, which has max = 308nm ( = 25,000), what concentration should be prepared if the desired absorbance is 0.5 at the maximum?
-
Indicate the types of transitions responsible for the absorptions of these compounds: Apax = 252 nm (e = 20,000) Aas - 325 nm ( = 180) a) A mux = 235 nm (e = 19,000) b) c) Amax = 299 nm ( = 20) d)...
-
Which of these compounds are expected to have an absorption maximum in the region of 200 to 400nm in their UV spectra? ) C-CH,CH, b) CH,CH,CH3 c) d) f) CH,CH,OCH,CH3 e) h) g)
-
Discuss with examples about financial innovation& how it develops banking industry?
-
Elaborate with real world examples about different financial markets that contributed towards growth of financial sector?
-
The following statement of financial position is for the partnership of Able, Brown, and Crown at November 1, 2018. Assets Liabilities Cash $ 20,000 Accounts payable $ 50,000 Other assets 180,000...

Study smarter with the SolutionInn App


