Hydrogen peroxide and the iodide ion react in acidic solution as follows: The kinetics of this reaction
Question:
Hydrogen peroxide and the iodide ion react in acidic solution as follows:
![]()
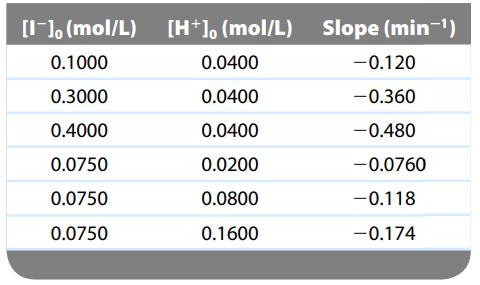
The kinetics of this reaction were studied by following the decay of the concentration of H2O2 and constructing plots of ln[H2O2] versus time. All the plots were linear and all solutions had [H2O2]0 = 8.0 × 10-4 mol/L. The slopes of these straight lines depended on the initial concentrations of I- and H+. The results follow:
The rate law for this reaction has the form
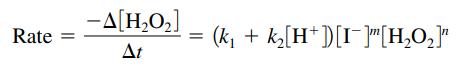
a. Specify the order of this reaction with respect to [H2O2] and [I-].
b. Calculate the values of the rate constants, k1 and k2.
c. What reason could there be for the two-term dependence of the rate on [H+]?
Step by Step Answer:

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste





