Solid NH 4 HS decomposes by the following endothermic process: a. What effect will adding more NH
Question:
Solid NH4HS decomposes by the following endothermic process:
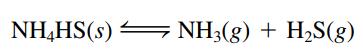
a. What effect will adding more NH3(g) have on the equilibrium?
b. What effect will adding more NH4HS(s) have on the equilibrium?
c. What effect will increasing the volume of the container have on the equilibrium?
d. What effect will decreasing the temperature have on the equilibrium?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





