Sulfur trioxide, SO 3 , is produced in enormous quantities each year for use in the synthesis
Question:
Sulfur trioxide, SO3, is produced in enormous quantities each year for use in the synthesis of sulfuric acid.
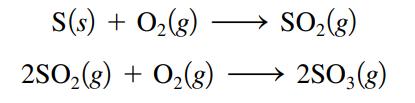
What volume of O2(g) at 350.οC and a pressure of 5.25 atm is needed to completely convert 5.00 g sulfur to sulfur trioxide?
Transcribed Image Text:
S(s) + 0₂ (8) + O₂(g) 2SO₂(g) SO₂(g) 2SO3(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The volume 500 g x 1 mol SO38409 g SO3 20 moles O21 mol O2 0...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
An ideal gas at 15.5 C and a pressure of 1.72 105 Pa occupies a volume of 2.81 m3. (a) How many moles of gas are present? (b) If the volume is raised to 4.16 m3 and the temperature raised to 28.2...
-
Air at a temperature of 30 C and a pressure of 100 kPa has a relative humidity of 80%. Calculate (a) The molal humidity of the air (b) Saturated molal humidity of this air if its temperature is...
-
5 liters of air with a humidity of 0.6, a temperature of 20 C and a pressure of 75 cm Hg it is pressed together to 2 liters at a temperature of 25 C. Calculate all missing state parameters, the...
-
Write a function my_ieee_2_dec(ieee), where icce is a string contains 64 char- acters of ones and zeros representing a 64-bit IEEE754 number. The output should be d, the equivalent decimal...
-
Adriana Santosh is in the business of selling antique furniture. As her company has increased significantly the Internet portion of their business, Adriana has determined that she does not need as...
-
What is a steam generator ?
-
Selenelion Ltd is a small manufacturing business. For the year ending 30 June 2024, the company achieved sales of $2 772 000 and a gross profit margin of 30%. Although satisfied with this result,...
-
Consider a venturi with a throat-to-inlet area ratio of 0.8, mounted on the side of an airplane fuselage. The airplane is in flight at standard sea level. If the static pressure at the throat is 2100...
-
A satellite is placed in a circular orbit to observe the surface of Mars from an altitude of 144 km. The equatorial radius of Mars is 3397 km. If the speed of the satellite is 3480 m/s, what is the...
-
This problem continues the Canyon Canoe Company situation from Chapter 6. Canyon Canoe Company has decided to open a new checking account at River Nations Bank during March 2019. Canyon Canoe...
-
A typical adult inhales 450 mL of air in any one breath. How many air particles are in a typical breath at 745 torr and 22C?
-
A 2.50-L container is filled with 175 g argon. a. If the pressure is 10.0 atm, what is the temperature? b. If the temperature is 225 K, what is the pressure?
-
Kross Company purchased a machine at a price of $100,000 by signing a note payable, which requires a single payment of $118,810 in 2 years. Assuming annual compounding of interest, what rate of...
-
Explain the difference between intrinsically motivated behavior and extrinsically motivated behavior?
-
What are the common sources of contemporary work stress? explain
-
The most recent financial statements for Bello, Inc., are shown here: Balance Sheet Debt Equity Income Statement Sales Costs Taxable income $38,600 Assets $140,000 26,500 $ 12,100 Total $140,000...
-
What is the output produced by the following code? int a=2, b=4; for (int i=0; i <9; i++) a+= 2; b+= 4; System.out.println(a+b);
-
Investment X has a 25% chance of producing a 20% return, a 50% chance of producing a 15% return, and a 25% chance of producing a return of -5%. What's Investment X's coefficient of variation?
-
Assuming that such a fly would be viable, what would be the sex of a fruit fly with the following chromosomal composition? A. One X chromosome and two sets of autosomes B. Two X chromosomes, one Y...
-
Shreemaya Hotel in !adore was facing a problem of low demand for its rooms due to off season. The Managing Director (MD) of the hotel, Mrs. Sakina was very worried. She called upon the Marketing...
-
Using any compounds that contain two carbon atoms or fewer, show a way to prepare a racemic mixture of (2R, 3R)- 2,3-dihydroxypentane and (2S,3S)-2,3-dihydroxypentane.
-
For each pair of compounds below, identify the more acidic compound: (a) (b) (c) (d) (e) (f) (g) (h) SH
-
Paclitaxel (marketed under the trade name TaxolTM) is found in the bark of the Pacific yew tree, Taxus berevifolia, and is used in the treatment of cancer: (a) Draw the enantiomer of paclitaxel. (b)...
-
Individual Retirement Account (IRA) Bonds Mutual fund Stocks Futures Defined contribution plans What is it? Level of Risk and Potential Return Minimum investment? Easy to start or stop?
-
1. A company purchased machinery in 2015 for $400,000. Its value in 2018 was $320,000. Assuming the resale value decreases exponentially, what will the value be in 2020? As a part of your solution,...
-
ROA of a company is 8.57%, Total assets end of the year of 2021 are $9.6 million, ROE is 14% and Profit margin of 19.9% what is the firms value of net income? and what is stockholders equity?

Study smarter with the SolutionInn App


