A balloon initially contains 40 m 3 of helium gas at atmospheric conditions of 100 kPa and
Question:
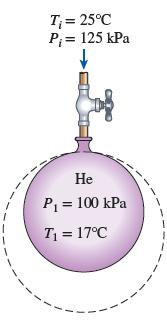
A balloon initially contains 40 m3 of helium gas at atmospheric conditions of 100 kPa and 17°C. The balloon is connected by a valve to a large reservoir that supplies helium gas at 125 kPa and 25°C. Now the valve is opened, and helium is allowed to enter the balloon until pressure equilibrium with the helium at the supply line is reached. The material of the balloon is such that its volume increases linearly with pressure. If no heat transfer takes place during this process, determine the final temperature in the balloon.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:





