For correct operation of the catalytic converter in an automobile, the airfuel ratio of the engine must
Question:
For correct operation of the catalytic converter in an automobile, the air–fuel ratio of the engine must be precisely controlled to near the stoichiometric value. This control is achieved using feedback from an O2 sensor located in the exhaust stream.
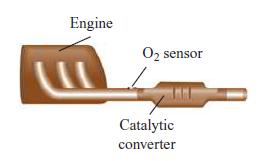
A. If the equivalent composition of gasoline is given by C8H15, determine the value of the stoichiometric air–fuel ratio (by mass). Assume that air is a simple mixture of O2 and N2 in molar proportions of 1:3.76.
B. Assuming complete combustion at stoichiometric conditions, determine the mole fractions of each constituent in the exhaust stream.
C. Determine the molar mass (apparent molecular weight) of the exhaust gas mixture.
Step by Step Answer:

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley





