A cylinder closed by a piston contains N moles of a diatomic gas characterised by U =
Question:
A cylinder closed by a piston contains N moles of a diatomic gas characterised by U = (5/2) NRT and by pV = NRT, as in exercise 4.1. The gas has a temperature T when it is brought in contact with a heat reservoir at temperature Text, causing an irreversible process to occur. The pressure p of the gas is equal to the constant pressure pext of the environment at all times, i.e. p = pext = const. Determine the amount of heat exchanged.
Numerical application:![]()
Data from in Exercise 4.1
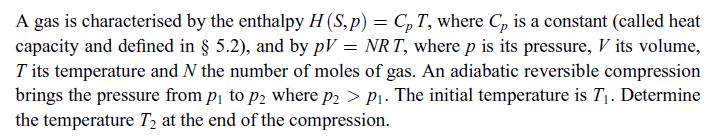
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





