A laboratory analysis shows that CO 2 becomes 10 % dissociated into CO and O 2 at
Question:
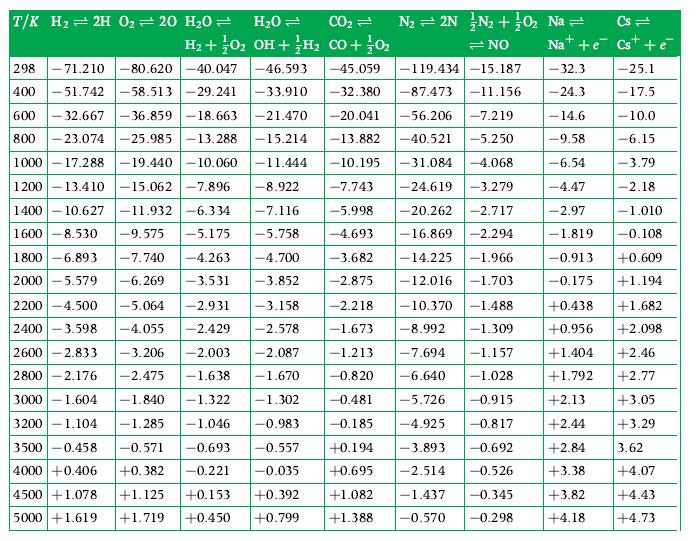
A laboratory analysis shows that CO2 becomes 10 % dissociated into CO and O2 at 2390 K if the total pressure is 1 atm. Obtain the equilibrium constant from this information and compare it to the value given in Table D.1.
Transcribed Image Text:
T/K H₂2H 0₂ = 20 H₂0 = H₂O = CO₂ = N₂ = 2N N₂ + 0₂ Na = Cs= H₂ + O2 OH + H₂ CO + 0₂ =NO Nat+e Cs + é -45.059 -32.3 -32.380 -87.473 -11.156 -24.3 -20.041 -56.206 -7.219 - 14.6 -13.882 -40.521 -5.250 -9.58 -10.195 -31.084 -4.068 -6.54 298 -71.210 -80.620-40.047 -46.593 400 -51.742-58.513 -29.241 -33.910 600 -32.667 -36.859-18.663 -21.470 800-23.074 -25.985 -13.288 -15.214 1000-17.288 -19.440 -10.060 -11.444 1200-13.410 -15.062 -7.896 -8.922 1400-10.627 -11.932-6.334 -7.116 1600 -8.530 -9.575 -5.175 -5.758 1800 -6.893 2000 -5.579 -4.47 -2.97 -7.743 -24.619 -3.279 -5.998 -20.262 -2.717 -4.693 -16.869 -2.294 -3.682 -14.225 -1.966 -2.875 -12.016 -1.703 -1.819 -7.740 -4.263 -4.700 -3.852 -6.269 -3.531 2200 -4.500 -5.064 -2.931 2400 -3.598 -4.055 -2.429 2600 -2.833 -3.206 -2.003 2800 -2.176 -2.475 -1.638 3000 -1.604 -1.840 -1.322 -2.218 -10.370 -1.488 -1.673 -8.992 -1.309 -7.694 -1.157 -6.640 -1.028 -5.726 -0.915 -4.925 -0.817 -3.893 -0.692 -2.514 -0.526 -0.345 -0.298 -3.158 -2.578 -2.087 -1.213 -1.670 -0.820 - 1.302 -0.481 -0.185 +0.194 +0.695 3200-1.104 -1.285 -1.046 -0.983 3500 -0.458 -0.571 -0.693 -0.557 4000 +0.406 +0.382 -0.221 -0.035 4500 +1.078 +1.125 +0.153 5000 +1.619 +1.719 +0.450 +0.392 +0.799 -119.434-15.187 +1.082 -1.437 +1.388. -0.570 -25.1 -17.5 -10.0 -6.15 -3.79 -2.18 -1.010 -0.108 -0.913 +0.609 -0.175 +1.194 +1.682 +2.098 +2.13 +2.44 +2.84 +3.38 +0.438 +0.956 +1.404 +2.46 +1.792 +2.77 +3.05 +3.29 3.62 +4.07 +3.82 +4.43 +4.18 +4.73
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (6 reviews)
The reaction can be written as CO CO 050 As the laboratory analysi...View the full answer

Answered By

Lilian Nyambura
Hi, am Lilian Nyambura, With extensive experience in the writing industry, I am the best fit for your writing projects. I am currently pursuing a B.A. in Business Administration. With over 5 years of experience, I can comfortably say I am good in article writing, editing and proofreading, academic writing, resumes and cover letters. I have good command over English grammar, English Basic Skills, English Spelling, English Vocabulary, U.S. English Sentence Structure, U.K. or U.S. English Punctuation and other grammar related topics. Let me help you with all your essays, assignments, projects, dissertations, online exams and other related tasks. Quality is my goal.
4.80+
378+ Reviews
750+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
A sample of O2 is collected over water in a 5.00 L container at 20C. If the total pressure is 688 torr, how many moles of O2 are collected?
-
In a gas mixture of O2 and N2, the total pressure is 2.66 atm and the partial pressure of O2 is 888 torr. What is the partial pressure of N2?
-
A sample of CO is collected over water in a 25.00 L container at 5C. If the total pressure is 0.112 atm, how many moles of CO are collected?
-
Ebrahim Patel is a wholesaler who uses the periodic inventory system to account for inventory. Transactions for February: 1 Bought inventory from Rich Traders for R5 000 on credit. 2 Sold inventory...
-
The town of Musicville has two residents: Bach and Mozart. The town currently fund sits free outdoor concert series solely from the individual contributions of these residents. Each of the two...
-
Andrew Finch, a stockbroker at Morgan Stanley Dean Witter & Co., assumed that his role in making Morgan Stanley the lead underwriter on the initial public offering (IPO) of Webmethods stock would...
-
Discuss under what circumstances a nurse has a duty to question a patients care.
-
Large Ltd. purchased 75% of Small Company on January 1, Year 1, for $600,000, when the statement of financial position for Small showed common shares of $400,000 and retained earnings of $100,000. On...
-
Pitch a recommendation to a company of your choosing with your prediction of the potential success or downfall of Facebook and the MetaVerse for social media marketing. Essentially, would you...
-
1. East Coast Yachts uses a small percentage of preferred stock as a source of financing. In calculating the ratios for the company, should preferred stock be included as part of the companys total...
-
A turboprop engine has the following characteristics: The engine propels an airplane at 640 km/hr at an altitude of 7600 m (?35 ?C, 38 kPa). Analyze the thermodynamic cycle on a per kg basis, making...
-
Propane (C 3 H 8 ) and oxygen in stoichiometric proportions react in a steady flow water-cooled burner. The reactants enter the burner at 305 K and leave the burner at 2 atm and emerge at 730 K. The...
-
Prenumbering of source documents helps to verify that a. multiple types of source documents have a unique identifier. b. all transactions have been recorded because the numerical sequence serves as a...
-
In the aftermath of the global financial crisis, U.S. government budget deficits increased dramatically, yet interest rates on U.S. Treasury debt fell sharply and stayed low for many years. Does this...
-
Which of the following resources may be used to pay for a long-term care stay in a nursing home that exceeds 100 days? A. Medicaid. B. Medicare. C. Medicare supplement insurance. D. Medigap insurance.
-
Under an endorsement form of split-dollar life insurance, the insureds spouse is which of the following? A. The owner of the life insurance contract. B. The beneficiary of the life insurance...
-
An SEP has which characteristic? A. Loans and hardship withdrawals are available. B. Age-weighting or cross-testing is permitted. C. Social Security integration is permitted. D. Employer matching is...
-
Angie and Pat Rice want to begin funding for their daughter Ashleys education. Ashley is in pre-K at the Wilson School. She will need a computer when she begins first grade in two years. How should...
-
Charles Ebo was terminated from his employment with QR Ltd. in July 20X6. In November 20X6, he began work as a commission salesperson for AP Ltd., a Canadian public corporation. Ebo has asked you to...
-
Sheldon and Leonard had a million-dollar idea. In order to make it happen, they have to do special research first. Only Kripke can help them in this matter. But Kripke is known to be the first-class...
-
Spreadsheet for standard deviation. Lets create a spreadsheet to compute the mean and standard deviation of a column of numbers in two different ways. The spreadsheet here is a template for this...
-
Use Table 4-1 for this exercise. Suppose that the mileage at which 10000 sets of automobile brakes had been 80% worn through was recorded. The average was 62700, and the standard deviation was 10 400...
-
Use the NORMDIST spreadsheet function to answer these questions about the brakes described in Exercise 4-C: (a) What fraction of brakes is expected to be 80% worn in less than 45 800 miles? (b) What...
-
Using the given field diagram of the three charges below, calculate the net Electric Field Strength (include angle) at the yellow point shown in the diagram. The charge values are shown, the distance...
-
Construct a simple basic circuit in which a 6 resistor is connected in series to the battery. Hover the ammeter over a wire near the battery to measure the current. Connect a voltmeter parallel to...
-
Consider a system composed of an ideal gas. (a) (2 points) Derive P(V), this is, pressure as a function of volume, for a reversible adiabatic process. State any assumptions. (b) (2 points) Consider...

Study smarter with the SolutionInn App


