An isolated system consists of two blocks made of the same substance (Fig. 3.9). The internal energies
Question:
An isolated system consists of two blocks made of the same substance (Fig. 3.9). The internal energies of blocks 1 and 2 are U1 = C1 T1 and U2 = C2 T2 where C1 and C2 are two positive constants. Two sides of the blocks face each other exactly. The area of each side is A and they are separated by a fixed air gap. We neglect the heat conductivity of the air in the gap. The radiative thermal power that block i exerts on block j, where i, j = 1, 2, is given by,
Figure 3.9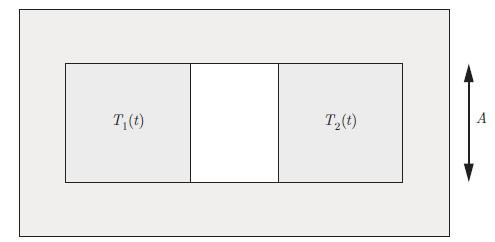
![]() where σ is a constant coefficient.
where σ is a constant coefficient.
(a) Determine the final temperature Tf of the system when it reaches equilibrium.
b) Derive the time evolution equation for T1 (t) and T2 (t).
c) Consider the particular case where C1 = C2 = C and the limit of small temperature variations, i.e. T1 (t) = Tf +ΔT1 (t) and T2 (t) = Tf +ΔT2 (t) with ΔT1 (t) ≪ Tf and ΔT2 (t) ≪ Tf at all times. Show that the temperature difference ΔT (t) = ΔT1 (t) − ΔT2 (t) is exponentially decreasing.
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





