Analyse the time evolution of a gas consisting of one substance that diffuses through a permeable wall.
Question:
Analyse the time evolution of a gas consisting of one substance that diffuses through a permeable wall. Thus, consider an isolated system containing N moles of gas, consisting of two subsystems of equal volumes separated by a fixed and permeable wall, with N1(t) moles of gas in subsystem 1 and N2(t) in subsystem 2. In order to be able to find the time evolution, model the chemical potentials in each subsystem by assuming that they are proportional to the amount of matter. In order to simplify the expressions in the solution, write,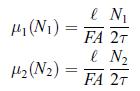 where τ > 0 will be identified as a specific diffusion time, F > 0 is the Fick diffusion coefficient and > 0 is a specific length. Initially, there are N0 moles in the subsystem 1, i.e. N1(0) = N0, and N − N0 moles in the subsystem 2, i.e. N2(0) = N − N0. Determine the evolution of the number of moles N1(t) and N2(t). Find the number of moles in each subsystem when equilibrium is reached.
where τ > 0 will be identified as a specific diffusion time, F > 0 is the Fick diffusion coefficient and > 0 is a specific length. Initially, there are N0 moles in the subsystem 1, i.e. N1(0) = N0, and N − N0 moles in the subsystem 2, i.e. N2(0) = N − N0. Determine the evolution of the number of moles N1(t) and N2(t). Find the number of moles in each subsystem when equilibrium is reached.
Step by Step Answer:

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet





