Consider the reaction of air to form a gaseous mixture containing N 2 , O 2 ,
Question:
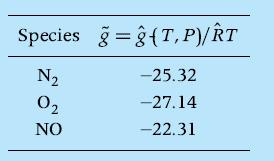
Consider the reaction of air to form a gaseous mixture containing N2, O2 , and NO and assume that the perfect gas law is applicable. Calculate the mole fractions of the species at equilibrium at T = 2000 K and P = 5 atm using the element potentials method. The nondimensional Gibbs function values of the pertinent species at these conditions are
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





