Given the set of experimental VLE data for a CO 2 /toluene mixture at T = 80C
Question:
Given the set of experimental VLE data for a CO2 /toluene mixture at T = 80°C of W. Morris and M. Donohue, “Vapor–liquid equilibria in mixtures containing carbon dioxide, toluene, and i-methylnaphthalene,” J. Chem. Eng. Data, 30, (3), 259–263, 1985,
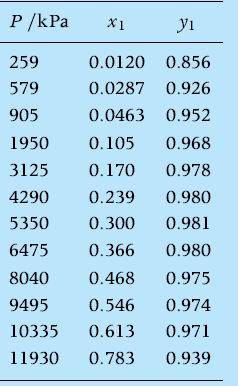
estimate the Henry’s constant. Use the result to calculate the bubble pressure at 400 K for a molar concentration of CO2 in the liquid phase of 0.015.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:





