The throttling calorimeter is a device for determining the thermodynamic state of a fluid in vaporliquid equilibrium.
Question:
The throttling calorimeter is a device for determining the thermodynamic state of a fluid in vapor–liquid equilibrium. The procedure to estimate the vapor fraction of the fluid consists in bleeding off a small amount of the two-phase fluid, throttle it through a valve, and take the temperature and pressure measurements as shown in the figure. Explain why P1 and T1 are not sufficient to fix the thermodynamic state of the wet fluid, and how P2 and T2 measurements allow state 1 to be determined. How much throttling (pressure drop caused by the valve) is necessary for this quality measurement technique to work? Do you see limitations in terms of measurable states?
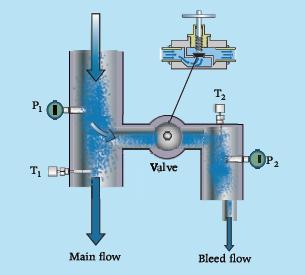
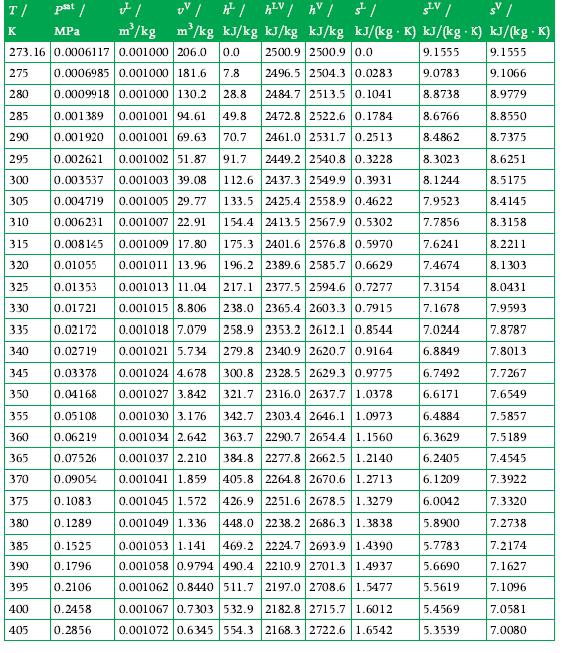
In the case that the fluid is water, calculate the quality if the readings of the calorimeter are
P1 = 10 bar, T1 = 179.9 °C,
P2 = 1.2 bar, T2 = 120 °C.
Refer to the tables in Appendix A.3 for data.
Data From A.3
Step by Step Answer:

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna





