The molecular weight of a polymer can be determined from its viscosity by the following relationship: Where
Question:
The molecular weight of a polymer can be determined from its viscosity by the following relationship:
Where [η] is the intrinsic viscosity of the polymer Mυ is the viscosity averaged molecular weight, and K and α are constants specific for the polymer. The intrinsic viscosity is determined experimentally be determining the efflux time, or the time it takes for the polymer solution to flow between two etched lines in a capillary viscometer, at several different concentration of dilute polymer, and extrapolating to infinite dilution. A plot of
-2.png)
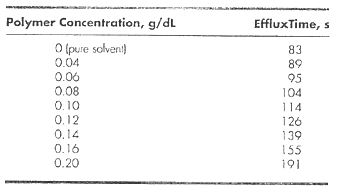
should yield a straight line, with a y intercept equal to [η]. The concentration of the polymer solution is c, t is the efflux time of polymer solution, and t0 is the efflux time of the solvent without polymer. Using the data below of efflux times for dilute solutions of polystyrene in methyl ethyl ketone at 25°C and the constants K = 3.9 x 10-4 and α = 0.58, find the molecular weight of the polystyrene sample.
Step by Step Answer:

Numerical Methods For Engineers
ISBN: 9780071244299
5th Edition
Authors: Steven C. Chapra, Raymond P. Canale





