The relative binding of the extra electron in the arsenic atom that replaces an atom in silicon
Question:
The relative binding of the extra electron in the arsenic atom that replaces an atom in silicon or germanium can be understood from a calculation of the first Bohr orbit of this electron in these materials. Four of arsenic's outer electrons form covalent bonds, so the fifth electron sees a singly charged center of attraction. This model is a modified hydrogen atom. In the Bohr model of the hydrogen atom, the electron moves in free space at a radius a0 given by
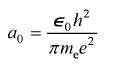
When an electron moves in a crystal, we can approximate the effect of the other atoms by replacing ?0 with ??0 and me with an effective mass for the electron. For silicon ??is 12 and the effective mass is about 0.2me. For germanium ??is 16 and the effective mass is about 0.1me. Estimate the Bohr radii for the outer electron as it orbits the impurity arsenic atom in silicon and germanium.
Step by Step Answer:

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry





