The speed of a molecule in a uniform gas at equilibrium is a random variable V whose
Question:
The speed of a molecule in a uniform gas at equilibrium is a random variable V whose probability distribution is given by where k is an appropriate constant and b depends on the absolute temperature and mass of the molecule. Find the probability distribution of the kinetic energy of the molecule W, where W = mV2/2.
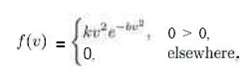
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Probability & Statistics For Engineers & Scientists
ISBN: 9780130415295
7th Edition
Authors: Ronald E. Walpole, Raymond H. Myers, Sharon L. Myers, Keying
Question Posted:





