What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M
Question:
What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M Cd(II) in 1.0 M ammonia if there is negligible current? Consider the following reactions and assume that nearly all Cd(II) is in the form Cd(NH3)42+.
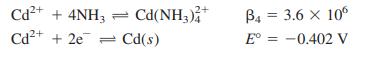
Transcribed Image Text:
Cd2+ + 4NH3 Cd(NH,)* 2+ B4 = 3.6 x 10 Cd2+ + 2e = Cd(s) E° = -0.402 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
When 9999 of CdII is reduced the form...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
A proton has an initial speed of 5.5 105 m/s. (a) What potential difference is required to bring the proton to rest? (b) What potential difference is required to reduce the initial speed of the...
-
Ti 3+ is to be generated in 0.10 M HClO 4 solution for coulometric reduction of azobenzene. At the counter electrode, water is oxidized, and O 2 is liberated at a pressure of 0.20 bar. Both...
-
Electrogravimetric analysis with control of the cathode potential is proposed as a means for separating Bi3+ and Sn2+ in a solution that is 0.250 M in each ion and buffered to pH 1.95. (a) Calculate...
-
Suppose in a given area there are three power plants, each of which emits SO 2 with different intensities. The abatement cost functions for each firm j are: (a) Set up the conditions for the socially...
-
LaPorta Company and Lott Corporation, two corporations of roughly the same size, are both involved in the manufacture of in-line skates. Each company depreciates its plant assets using the...
-
John Wiley Publishing Company publishes an operations management textbook that is scheduled for a revision. The book has been moderately successful, but each year more new books enter the market,...
-
Suppose \(V=V(S)\). Find the most general solution of the Black-Scholes equation.
-
The CFO of Turing Corporation is very uncomfortable with its current risk exposure related to the possibility of business disruptions. Specifically, Turing is heavily involved in e- business, and its...
-
Assume that P = Tshs 1 0 0 , 0 0 0 , i = 1 2 % , and n = 3 years. Find FV when interest is compounded: ( a ) annually
-
A company produces several products which pass through the two production departments in its factory. These two departments are concerned with filling and sealing operations. There are two service...
-
The figure shows the behavior of Pt and Ag cathodes at which reduction of H 3 O + to H 2 (g) occurs. Explain why the two curves are not superimposed. Pt Ag 0.5 -0.2-0.3-0.4-0.6-0.7-0.8-0.9 E(V vs....
-
Electroplating efficiency. Nickel was electrolytically plated onto a carbon electrode from a bath containing 290 g/L NiSO 4 6H 2 O, 30 g/L B(OH)3, and 8 g/L NaCl at - 1.2 V vs. Ag | AgCl. The most...
-
Prove each identity, assuming that S and E satisfy the conditions of the Divergence Theorem and the scalar functions and components of the vector fields have continuous second-order partial...
-
Josh is graduating at the end of the academic year with a BS degree in engineering. He already has an offer with a good company for $58,000. He has learned that those who continue along a technical...
-
Your money is tied up and you need to borrow \($10\),000. The following two alternatives are available at different banks: (1) Pay \($3\),311.61 at the end of each year for 5 years, starting at the...
-
Med Diagnostics Inc. borrowed \($200\),000 from a lender for a new blood analyzer module to improve accuracy and consistency of its tests. The rate was 6 percent, 2 percent above the prime rate. The...
-
Recall Problem 34. Shortly before Jerry got under way with his job and investment plan, he met Jennifer. All of a sudden, he decided to put the retirement plan on hold, reasoning that 40 years is a...
-
What is the effective annual interest rate for 5 percent compounded (a) semiannually, (b) every 4 months, (c) quarterly, (d) every other month, (e) monthly?
-
North America, Greenland, and Eurasia fit quite well together in reconstructing Laurasia, but there is no space available for Iceland. Why is the omission of Iceland from Laurasia reasonable?
-
What is a lobbyist in US? How did this term emerge?
-
An unknown sample of Ni 2+ gave a current of 2.36 A in an electrochemical analysis. When 0.500 mL of solution containing 0.028 7 M Ni 2+ was added to 25.0 mL of unknown, the current increased to 3.79...
-
A solution was prepared by mixing 5.00 mL of unknown element X with 2.00 mL of solution containing 4.13 g of standard element S per milliliter, and diluting to 10.0 mL. The signal ratio in atomic...
-
In Figure 5-6, the x-intercept is 2.89 mM and its standard deviation is 0.098 mM. Find the 90% and 99% confidence intervals for the intercept. Figure 5-6
-
The government standard on radiation from electrical devices is 10mW/cm2 (maximum). Assume a laptop computer is found to radiate more than this standard. The electric field inside is measured to be 5...
-
A 2 2 . 8 - m deep pool is filled with oil of density 8 9 5 kg / m ^ 3 . What is the gauge pressure at the bottom of the pool? Part B: What is the force from the okl that exerts on a 0 . 3 5 m x 0 ....
-
Clark was driving his four-wheeler 15 meters per second on a dirt road. It had just rained and there were massive mud puddles on the road. He hits on and it slows him to a speed of 3.7 meters per...

Study smarter with the SolutionInn App


