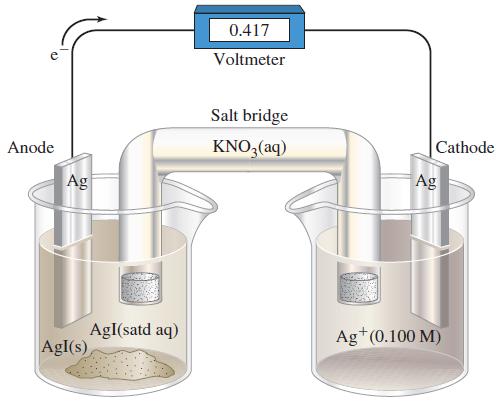
Assume that the volume of each solution in Figure 19-22 is 100.0 mL. The cell is operated
Question:
Assume that the volume of each solution in Figure 19-22 is 100.0 mL. The cell is operated as an electrolytic cell, using a current of 0.500 A. Electrolysis is stopped after 10.00 h, and the cell is allowed to function as a voltaic cell. What is Ecell at this point?
Figure 19-22
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





