Two equations can be written for the dissolution of Mg(OH) 2 (s) in acidic solution. (a) Explain
Question:
Two equations can be written for the dissolution of Mg(OH)2(s) in acidic solution.
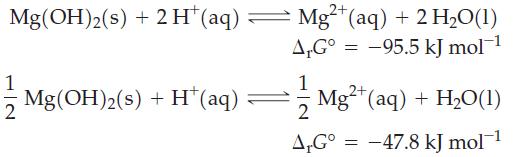
(a) Explain why these two equations have different ΔrG° values.
(b) Will K for these two equations be the same or different? Explain.
Transcribed Image Text:
2+ Mg(OH)2(s) + 2H* (aq) — Mg²+ (aq) + 2 H₂O(1) A.G° -95.5 kJ mol-¹ 1 Mg(OH)2(s) + H* (aq) 1 2 A,Gº = 2+ Mg²+ (aq) + H₂O(1) -47.8 kJ mol-¹ =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a The two equations have different rG values because they represent different paths for the same chemical reaction The first equation shows the direct ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In Section 11.3.1, we found the least squares estimators of α and β by a two-stage minimization. This minimization can also be done using partial derivatives. (a) Compute...
-
Water flows between the North American Great Lakes as depicted in Fig. P13.12. Based on mass balances, the following differential equations can be written for the concentrations in each of the lakes...
-
The schematic diagram of the solution process as the net sum of three steps in Figure 13.4 does not show the relative magnitudes of the three components because these will vary from case to case. For...
-
At the current year-end, a company shows the following unadjusted balances for selected accounts. a. After an analysis of future sales discounts, the company estimates that the Allowance for Sales...
-
The controller of Trenshaw Company wants to improve the companys control system by preparing a month-by-month cash budget. The following information is for the month ending July 31, 2014. June 30,...
-
In Exercise 17 of Section 7.3 a techniquewas outlined to prove that the Gauss-Seidel method converges when A is a positive definite matrix. Extend this method of proof to show that in this case there...
-
The Forrester Company's income statement and comparative balance sheets as of December 31 of 2019 and 2018 are shown below: Cash dividends of \(\$ 31,000\) were declared and paid during 2019. Plant...
-
Formworks Company prepares monthly budgets. The current budget plans for a September ending inventory of 15,000 units. Company policy is to end each month with merchandise inventory equal to a...
-
Kinder Soaps Sdn. Bhd. is planning to launch its new soap, The Main Event, in Malaysia for 2022. Based on the current market condition, the factory plans to produce 20,000 pieces with which the...
-
Currently, CO 2 is being studied as a source of carbon atoms for synthesizing organic compounds. One possible reaction involves the conversion of CO 2 to methanol, CH 3 OH. With the aid of data from...
-
For the reaction 2 SO 2 (g) + O 2 (g) 2 SO 3 (g), Kc = 2.8 x 10 2 M -1 at 1000 K. (a) What is r G at 1000 K? (b) If 0.40 mol SO 2 0.18 mol O 2 , and 0.72 mol SO 3 are mixed in a 2.50 L flask at...
-
Determine whether the following series converge absolutely or conditionally, or diverge. 3 k=1
-
In this Essay, will demonstrate that have understood this content and that you can apply it to your own life and future profession by selecting and exploring six to eight (6-8) different...
-
he management of an oil company is trying to decide whether to drill for oil in a particular field in the Gulf of Mexico. It costs the company $600 thousand to drill in the selected field. The...
-
The following information was extracted from the first-year absorption-based accounting records of Enigma Corporation Total fixed costs incurredP100, 000Total variable costs incurred50, 000Total...
-
Watch this 9-11 Frontline video that links the newsworthy 9-11 event to the news events in the video. https://www.pbs.org/wgbh/frontline/documentary/america-after-9-11/ STEP 2 - Select either the...
-
Find the first derivative d and d x . Show all steps and simplify answer. and = x 2 + 1 x 2 - 1
-
A sporting goods manufacturer was asked to sponsor a local boy in two fishing tournaments. They claim the probability that he will win the first tournament is 0.4. If he wins the first tournament,...
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
Weighted-average method, assigning costs (continuation of 17-19). For the data in Exercise 17-19, summarize total costs to account for, calculate cost per equivalent unit for direct materials and...
-
FIFO method, equivalent units. Refer to the information in Exercise 17-19. Suppose the Assembly Division at Fenton Watches, Inc., uses the FIFD method of process costing instead of the...
-
FIFO method, assigning costs (continuation of 11-21). For the data in Exercise 17-19, use the FIFO method to summarize total costs to account for, calculate cost per equivalent unit for direct...
-
You are given the following data about the market of options on the CAC 40 index: Also, consider that the cost of a ZCB with expiry in 5 years is 97 Euros. You want to structure a certificate with...
-
1. How we Mitigate identified intellectual property (IP) risks associated with IP infringement in order to ensure business viability within both the domestic and international target markets ? Q2....
-
Lydia is a 50-year-old female, non-smoker, married with a daughter aged 10. Her annual gross income is $70,000. Has an existing mortgage of $420,000 with 12 more years of amortization. She has a...

Study smarter with the SolutionInn App


