A 2.0 mol sample of an ideal monatomic gas undergoe the reversible process shown in Figure, the
Question:
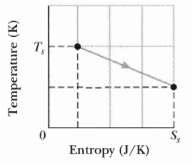
A 2.0 mol sample of an ideal monatomic gas undergoe the reversible process shown in Figure, the scale of the vertical axis is set by Ts = 400.0K and the scale of the horizontal axis is set by Ss = 20.0.J/K.
(a) How much energy is absorbed as heat by the gas?
(b) What is the change in the internal energy of the gas?
(c) How much work is done by the gas?
Transcribed Image Text:
T, Entropy (J/K) Temperature (K)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a It is possible to motivate starting from Eq 203 the notion that heat may be ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Fundamentals of Physics
ISBN: 978-0471758013
8th Extended edition
Authors: Jearl Walker, Halliday Resnick
Question Posted:
Students also viewed these Thermodynamics questions
-
A 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure P22.62. The process A B is a reversible isothermal expansion. Calculate (a) the net work done by the gas, (b)...
-
A 2.50 mol sample of an ideal gas expands reversibly and isothermally at 360 K until its volume is doubled. What is the increase in entropy of the gas?
-
A 1.00-mol sample of an ideal monatomic gas is at an initial temperature of 300 K. The gas undergoes an is volumetric process acquiring 500 J of energy by heat. It then undergoes an isobaric process...
-
Examine your own organisation, or one that you know. What forms of pre-departure training does it offer?
-
For the given in Problem 4: (a) Recalculate g if ti = 20 percent and ai = 0 6. (b) What general conclusion can you reach about the relationship between g and t from your answer to Problem 4 in...
-
A major automobile manufacturer located in Georgetown, Kentucky, has two certified vendors that produce brake pads with the following information. Audubon Manufacturing is located in Columbus, Ohio,...
-
When should benchmarking be preferred to direct estimates of the magnitudes of benefits? When should direct estimates be preferred? Is it appropriate to use both?
-
Kelley Company has completed Octobers sales and purchases journals (Shown below). a. Total and post the journals to T accounts for the general ledger and the accounts receivable and accounts payable...
-
A. Can we use human capital theory to explain "Age-Discrimination"? If so, how? B. Why do some firms prefer to hire younger workers, especially for entry level positions? C. Why do some firms...
-
Wardell Company purchased a mini computer on January 1, 2019, at a cost of $48,500. The computer has been depreciated using the straight-line method over an estimated five-year useful life with an...
-
In Figure where V23 = 3.00V1, n moles of a diatomic ideal gas are taken through the cycle with the molecules rotating but not oscillating. What are? (a) p2/p1 (b) p3/p1 and (c) T3/T1 For path 1 ?? 2,...
-
A 10 g ice cube at 10oC is place in a lake whose temperature is 15oC. Calculate the change in entropy of the cube lake system as the ice cube comes to thermal equilibrium; with the lake. The...
-
Wright Companys Cash account shows a $27,500 debit balance and its bank statement shows $25,800 on deposit at the close of business on May 31. Prepare its bank reconciliation using the following...
-
Calculate AB InBev's expected abnormal NOPATs between 2009 and 2018 based on the information. How does the implied trend in abnormal NOPAT compare with the general trends in the economy? Assume that...
-
Use the sample data provided in the scenario to determine the parcel quantities. Each delivery costs the business: small parcel $12.10, large parcel $15.20, documents $8.50, all for standard...
-
A W21x57 floor beam supports a 5-inch-thick reinforced concrete slab with an effective width b of 75 inches. Sufficient shear connectors are provided to make the beam fully-composite. The concrete...
-
Suppose 8 = 1/3. Before the game starts, you tell the players that you will offer each of them money m > 0 at each period both of them cooperate. The new per period payoff matrix is as follows: D...
-
The graph below illustrates the following. When the market is served by six competitive firms, each firm will be producing 20 units. However, a natural monopoly that can emerge on this market can...
-
Investment Guru provides investment advice to customers for fees. On 30 June 2019, it completed its first year of operations. Some of the ledger account balances of the business, before any yearend...
-
Show, if u(x, y) and v(x, y) are harmonic functions, that u + v must be a harmonic function but that uv need not be a harmonic function. Is e"e" a harmonic function?
-
Space probes may be separated from their launchers by exploding bolts. (They bolt away from one another.) Suppose a 4800-kg satellite uses this method to separate from the 1500-kg remains of its...
-
A swimming duck paddles the water with its feet once every 1.6 s, producing surface waves with this period. The duck is moving at constant speed in a pond where the speed of surface waves is 0.32...
-
Moving Source vs. Moving Listener. (a) A sound source producing 1.00-kHz waves moves toward a stationary listener at one-half the speed of sound. What frequency will the listener hear? (b) Suppose...
-
A car alarm is emitting sound waves of frequency 520 Hz. You are on a motorcycle, traveling directly away from the car. How fast must you be traveling if you detect a frequency of 490 Hz?
-
Details of the capital structure of Webber Ltd. appear below: Bonds Number issued Coupon rate Interest payments Years to maturity 27,000 6% semi-annually 10 $90 Current price Preferred shares Number...
-
An Ice cream company has commissioned an ice sculpture for their end of year celebration. The ice sculpture is in the shape of a giant ice cream cone-the shape can be thought of as a cone with one...
-
1. A soft drink vendor at a popular beach resort analyzes his sales records and finds that if he seels x cans of pop in one day, his profit in dollars is given by the function P(x) = -0.012+3x-80....

Study smarter with the SolutionInn App


