A liquid mixture consisting of 100 kmol of 60 mol% benzene, 25 mol% toluene, and 15 mol%
Question:
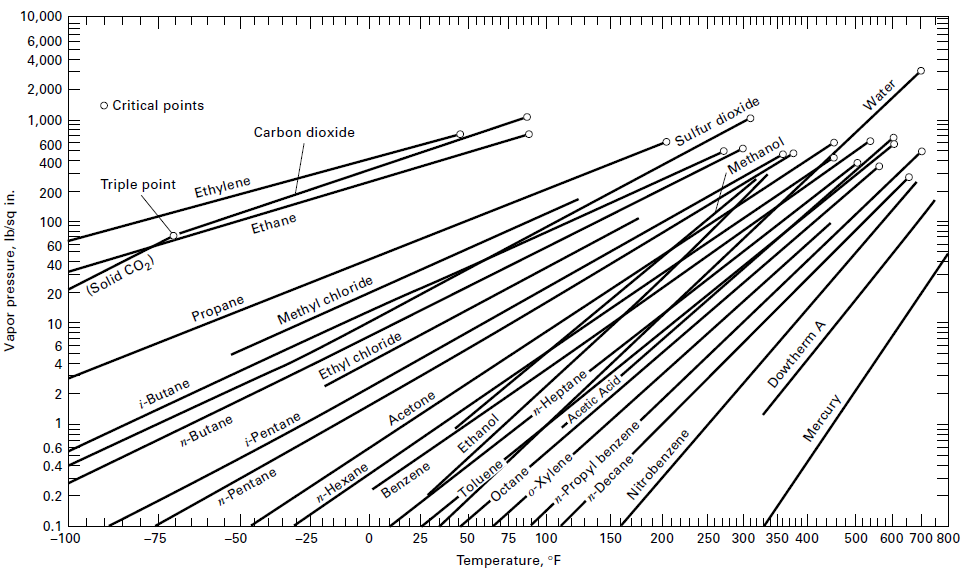
A liquid mixture consisting of 100 kmol of 60 mol% benzene, 25 mol% toluene, and 15 mol% o-xylene is flashed at 1 atm and 100°C.
(a) Compute the amounts of liquid and vapor products and their composition.
(b) Repeat the calculation at 100°C and 2 atm.
(c) Repeat the calculation at 105°C and 0.1 atm.
(d) Repeat the calculation at 150°C and 1 atm. Assume ideal solutions and use the vapor pressure curves of Figure for benzene and toluene. For o-xylene, draw a vapor pressure line that goes through the points (100.2oC, 200 torr) and (144oC, 760torr).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





