A liquid stream consisting of 12.5 mole% n-butane and the balance a heavy nonvolatile hydrocarbon is fed
Question:
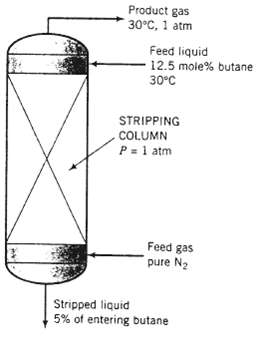
A liquid stream consisting of 12.5 mole% n-butane and the balance a heavy nonvolatile hydrocarbon is fed to the top of a stripping column, where it is contacted with an upward-flowing stream of nitrogen. The residual liquid leaves the bottom of the column containing all of the heavy hydrocarbon. 5% of the butane entering the column, and a negligible amount of dissolved nitrogen.
(a) The highest possible butane mole fraction in the exiting gas would be that in equilibrium with the butane in the entering liquid (a condition that would require an infinitely tall column to achieve). Using Raoult's law to relate the mole fractions of butane in the entering liquid and exiting gas, calculate the molar feed stream ratio (mol gas fed/mol liquid fed) corresponding to this limiting condition.
(b) Suppose the actual mole fraction of butane in the exit gas is 80% of its theoretical maximum value and the percentage stripped (95%) is the same as in part (a). Calculate the ratio (mol gas fed/mol liquid fed) for this case.
(c) Increasing the nitrogen feed rate for a given liquid feed rate and butane recovery decreases the cost of the process in one way and increases it in another. Explain. What would you have to know to determine the most cost-effective value of the gas/liquid feedratios?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





