A mixture of chloroform (CHC13) and acetic acid at 18oC and 1 atm (101.3 kPa) is to
Question:
A mixture of chloroform (CHC13) and acetic acid at 18oC and 1 atm (101.3 kPa) is to be extracted with water to recover the acid.(a) Forty-five kilograms of a mixture containing 35 wt% CHC13 and 65 wt% acid is treated with 22.75 kg of water at 18oC in a simple one-stage batch extraction. What are the compositions and weights of the raffinate and extract layers produced?(b) If the raffinate layer from the above treatment is extracted again with one-half its weight of water, what will be the compositions and weights of the new layers?(c) If all the water is removed from this final raffinate layer, what will its composition be?Solve this exercise using the following equilibrium data to construct one or more of the types of diagrams inFigure.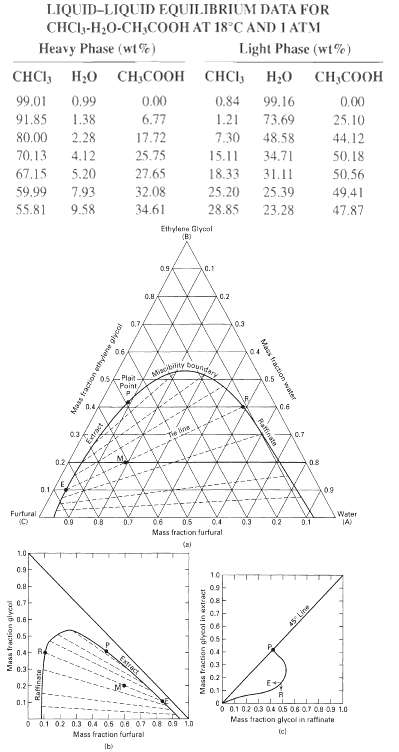
Step by Step Answer:






