Acetaldehyde is synthesized by the catalytic dehydrogenization of ethanol: C 2 H 5 OH ? CH 3
Question:
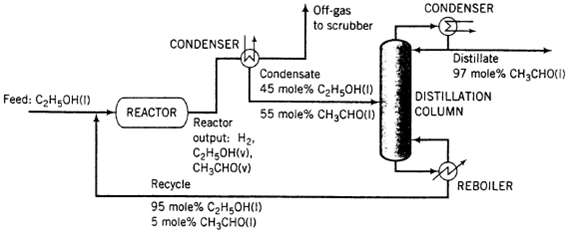
Acetaldehyde is synthesized by the catalytic dehydrogenization of ethanol: C2H5OH ? CH3CHO + H2?Fresh feed (pure ethanol) is blended with a recycle stream (95 mole% ethanol and 5% acetaldehyde), and the combined stream is heated and vaporized, entering the reactor at 280?C. Gases leaving the reactor are cooled to ?40?C to condense the acetaldehyde and un-reacted ethanol. Off-gas from the condenser is sent to a scrubber, where the uncondensed organic compounds are removed and hydrogen is recovered as a by-product. The condensate from the condenser, which is 45 mole% ethanol is sent to a distillation column that produces a distillate containing 97 mole% acetaldehyde and a bottoms product that constitutes the recycle blended with fresh feed to the process. The production rate of the distillate is 1000 kg/h. The pressure throughout the process may be taken as 1 atm absolute.
(a) Calculate the molar flow rates (k mol/h) of the fresh feed, the recycle stream, and the hydrogen in the off-gas. Also determine the volumetric flow rate (m3/h) of the feed to the reactor. (Suggestion: Use Raoult?s law in the analysis of the condenser.)
(b) Estimate (I) the overall and single-pass conversions of ethanol and (ii) me rates (k mol/h) at which ethanol and acetaldehyde are sent to the scrubber.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





