Ammonia is oxidized with air to form nitric oxide in the first step of the production of
Question:
Ammonia is oxidized with air to form nitric oxide in the first step of the production of nitric acid. Two principal reactions occur:
4 NH3 + 5O2 ? 4NO + 6H2O
2 NH3?+ 3/2 O2?? N2?+ 3H2O
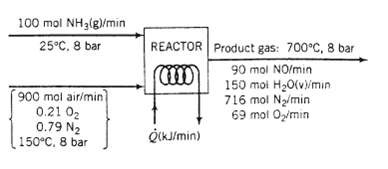
A flowchart of the reactor follows.
(a) Taking elemental species [N2 (g), H2?(g), O2?(g)] at 25?C as references, prepare and fill in an inlet? outlet enthalpy table.
(b) Calculate the required rate of heat transfer to or from the reactor in kW.
(c) What would have been different in your calculations and results in parts (a) and (b) if you had taken molecular species as references in part (a)?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





