An Equimolar liquid mixture of n-pentane and n-hexane at 80?C and 5.00 atm is fed into a
Question:
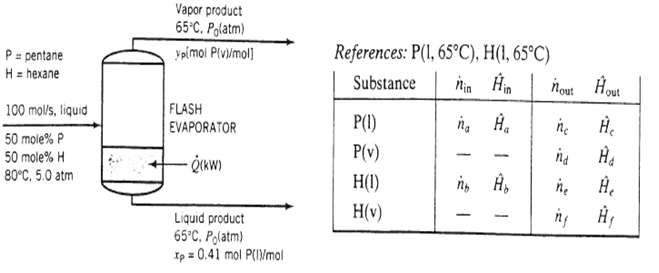
An Equimolar liquid mixture of n-pentane and n-hexane at 80?C and 5.00 atm is fed into a flash evaporator at a rate of 100.0mol/s. When the feed is exposed to the reduced pressure in the evaporators a substantial amount is vaporized. The temperature in the tank is maintained at 65?C by adding heat. The vapor and liquid phases, which are in equilibrium with each other, are separated and discharged as separate streams, the liquid product stream contains 41.0 mole% pentane A flowchart and an inlet?outlet enthalpy table for the process are given below.
(a) Using Raoult?s law for vapor?liquid equilibrium calculations, calculate (i) the system pressure, P0(atm), (ii) the mole fraction of pentane in the vapor product, yp, (iii) the volumetric flow rate of the vapor product. V (L/s), and (iv) the fractional vaporization of pentane, f (mol vaporized/mol fed).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





