An equimolar solution of benzene and toluene is totally evaporated at a constant temperature of 90C. What
Question:
An equimolar solution of benzene and toluene is totally evaporated at a constant temperature of 90°C. What are the pressures at the beginning and end of the vaporization process? Assume an ideal solution and use the vapor pressure curves ofFigure.
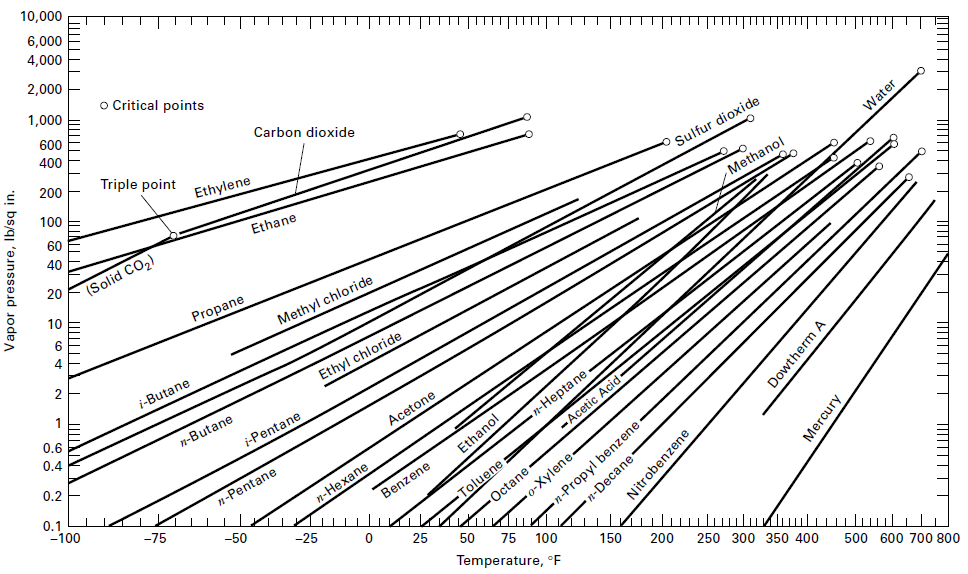
Transcribed Image Text:
10,000 6,000 4,000 2,000 П| o Critical points 1,000 600 400 Carbon dioxide 200 Triple point TП 100 Ethylene 60 40 Ethane Sulfur dioxide Water 20 (Solid CO2) Methanol 10 Propane 4 Methyl chloride i-Butane Ethyl chloride 0.6 0.4 n-Butane i-Pentane 0.2 Acetone n-Hepta ne 0.1 -100 -Acețic Acid- n-Pentane Ethanol Octane -o-Xylene- n-Propyl benzene п-Нехапe -75 KIn ulu Toluene: Benzene -50 -25 25 75 100 Temperature, °E 150 200 250 300 350 400 500 600 700 800 Vapor pressure, Ib/sqir ШІ n-Decane Nitrobenzene Dowtherm A Mercury
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (8 reviews)
Substitution of Raoults law Eq 3 in Table 23 ...View the full answer

Answered By

Sinmon Warui Kamau
After moving up and down looking for a job, a friend introduced me to freelance writing. I started with content writing and later navigated to academic writing. I love writing because apart from making a living out of it, it is also a method of learning and helping others to learn.
5.00+
40+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Chemical Engineering questions
-
A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash tank. The pressure in the unit may be adjusted to any desired value, and the heat input may...
-
A liquid mixture of benzene and toluene is to be separated in a continuous single-stage equilibrium flash tank. The pressure in the unit may be adjusted to any desired value, and the heat input may...
-
A vertical flat plate is maintained at a constant temperature of 120oF and exposed to atmospheric air at 70oF. At a distance of 14 in. from the leading edge of the plate the boundary layer thickness...
-
Under what circumstances should derived associations be used?
-
Refer to E8-10. Deere & Company reports in the notes to its financial statements that its trade accounts and notes receivable have significant concentrations of credit risk in the agricultural,...
-
Write code that prints the values stored in an array called names backwards.
-
Consider a stock price \(S\) governed by the geometric Brownian motion process (a) Using \(\Delta t=1 / 12\) and \(S(0)=1\), simulate several (i.e., many) years of this process using either method,...
-
During its first year of operations, Eastern Data Links Corporation entered into the following transactions relating to shareholders' equity. The articles of incorporation authorized the issue of 8...
-
Image transcription text 4) You've landed a spot on the operations team for the Europa Clipper mission! (htt s: www.' |.nasa. ov missions euro a-cli er or httpsz?europahasagovf ]. In addition to the...
-
Ellerson Company provided the following information for the last calendar year: Beginning inventory: Direct materials ...... $68,000 Work in process ...... 29,400 Finished goods ...... 43,200 Ending...
-
The following mixture is introduced into a distillation column as saturated liquid at 1.72MPa. Calculate the bubble-point temperature using the K-values of Figure. Compound kmol/h Ethane 1.5 Propane...
-
The following equations are given for the liquid-phase activity coefficients of the water (W)-acetic acid (A) system. Find the dew point and bubble point of a mixture of composition xw = 0.5, xA =...
-
A _______ is a genetic unit consisting of one or more populations of organisms that usually closely resemble one another physically and physiologically.
-
Define what a creative brief is and how it is developed and used.
-
What characteristics of risk affect the perception of a particular risk?
-
What is message framing? Give an example of a gain-framed appeal and a loss-framed appeal, and when you would use each.
-
What is a creative strategy statement? What are the main areas that make up a creative strategy? Define and explain each section.
-
How are theories/models of persuasion in health communication different from behavior change theories/models?
-
How is a dry appraisal well accounted for? a. Charged to dry hole expense b. Remains capitalized so long as a subsequent appraisal well is either planned or underway c. Capitalized if other...
-
A sample statistic will not change from sample to sample. Determine whether the statement is true or false. If it is false, rewrite it as a true statement.
-
Define the Sortino and Omega ratios and discuss their value in assessing ETF performance.
-
It is required to absorb 96% of the benzene from a gas stream with absorption oil in a sieve-tray column at a nominal pressure of 1 atm. The feed conditions are as follows: Tray geometry is as...
-
A ternary mixture of methanol, ethanol, and water is distilled in a sieve-tray column to obtain a distillate with not more than 0.01 mol% water. The feed to the column is as follows: Flow rate,...
-
A bubble-cap tray absorber is designed to absorb 40% of the propane from a rich gas at 4 atm. The specifications for the entering rich gas and absorbent oil are as follows: (a) Determine the number...
-
Create a segmentation, selection and focus model that allows the PUMA company in Mexico to determine its customer, where to find it and how to generate value given its model. 7) Selection of the most...
-
Determine if the following values are zeroes polynomial. 4 3 f(x) = x + x-18x - 16x + 32 x+1, +2, +4 -
-
Cali Windows is a small company that installs windows. Its cost structure is as follows: (Click the icon to view the cost structure.) Calculate (a) the breakeven point in units and revenues and (b)...

Study smarter with the SolutionInn App


