Chemical equilibrium and analysis of a mixture. (Warning! This is a long problem.) A remote optical sensor
Question:
Chemical equilibrium and analysis of a mixture. (Warning! This is a long problem.) A remote optical sensor for CO2 in the ocean was designed to operate without the need for calibration.
.png)
The sensor compartment is separated from seawater by a silicone membrane through which CO2, but not dissolved ions, can diffuse. Inside the sensor, CO2 equilibrates with HCO-3 and CO32-. For each measurement, the sensor is flushed with fresh solution containing 50.0 M bromothymol blue indicator (NaHIn) and 42.0 M NaOH. All indicator is in the form HIn- or In2- near neutral pH, so we can write two mass balances: (1) [HIn-] [In2-] FIn 50.0 M and (2) [Na+] FNa = 50.0μM + 42.0 μM + 92.0 μM. HIn- has an absorbance maximum at 434 nm and In2- has a maximum at 620 nm. The sensor measures the absorbance ratio RA = A620/A434 reproducibly without need for calibration. From this ratio, we can find [CO2(aq)] in the seawater as outlined here:
(a) From Beer’s law for the mixture, write equations for [HIn-] and [In2-] in terms of the absorbances at 620 and 434 nm (A620 and A434). Then show that
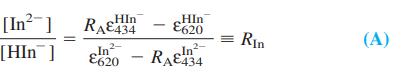
(b) From the mass balance (1) and the acid dissociation constant KIn, show that
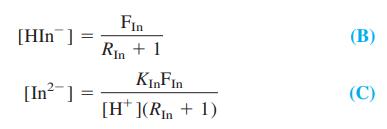
(c) Show that
![]()
(d) From the carbonic acid dissociation equilibria, show that
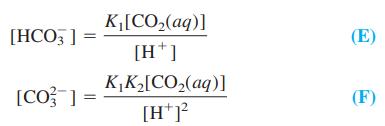
(e) Write the charge balance for the solution in the sensor compartment. Substitute in expressions B, C, E, and F for [HIn-], [In2-], [HCO3-], and [CO3 2-].
(f) Suppose that the various constants have the following values:
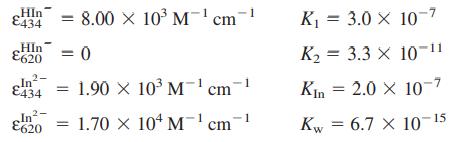
From the measured absorbance ratio RA = A620/A434 2.84, find [CO2(aq)] in the seawater.
(g) Approximately what is the ionic strength inside the sensor compartment? Were we justified in neglecting activity coefficients in working this problem?
Step by Step Answer:






