Dehydration of natural gas is necessary to prevent the formation of gas hydrates, which can plug valves
Question:
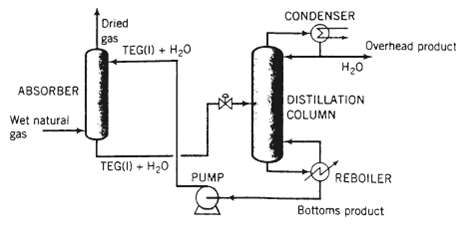
Dehydration of natural gas is necessary to prevent the formation of gas hydrates, which can plug valves and other components of a gas pipeline, and also to reduce potential corrosion problems. Water removal can be accomplished as shown in the following schematic diagram:
Natural gas containing 80lbm H2O/106 SCF gas [SCF = ft3 (STP)] enters the bottom of an absorber at a rate of 4.0 x 106 SCF/day. A liquid stream Containing triethylene glycol (TEG.. molecular weight 150.2) and a small amount of water is fed to the top of the absorber. The absorber operates at 500psia and 90?F. The dried gas leaving the absorber contains 10lbm H2O/10? SCF gas. The solvent leaving the absorber, which contains all the TEG?water mixture fed to the column plus all the water absorbed from the natural gas, goes to a distillation column. The overhead product stream from the distillation column contains only liquid water. The bottoms product stream, which contains TEG and water, is the stream recycled to the absorber.
(a) Draw and completely label a flowchart of this process. Calculate the mass flow rate day i and volumetric flow rate (ft3/day) of the overhead product from the distillation column.
(b) The greatest possible amount of dehydration is achieved if the gas leaving the absorption column is in equilibrium with the solvent entering the column. If the Henrys law constant for water in TEG at 90?F is 0.398psia/mol fraction, what is the maximum allowable mole fraction of water in the solvent fed to the absorber?
(c) A column of infinite height would be required to achieve equilibrium between the as and liquid at the top of the absorber. For the desired separation to be achieved in practice the mole fraction of water in the entering solvent must be less than the value calculated in part (b). Suppose it s 80% of that value and the flow rate of TEG in the re-circulating solvent is 37lbm TEG lbm. Water absorbed in the column. Calculate the flow rate (lbm/day) of the solvent stream entering the absorber and the mole fraction of water in the solvent stream leaving the absorber.
(d) What is the purpose of the distillation column in the process??
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





