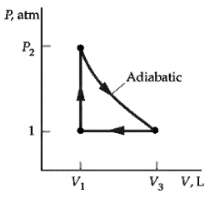
In the cycle shown in figure 1 mol of an ideal gas (? = 1.4) is initially
Question:
In the cycle shown in figure 1 mol of an ideal gas (? = 1.4) is initially at a pressure of 1atm and a temperature of 0oC. The gas is heated at constant volume to t2 = 150oC and is then expanded adiabatically until its pressure is again 1 atm. It is then compressed at constant pressure back to its original state. Find
(a) The temperature t3 after the adiabatic expansion,
(b) The heat entering or leaving the system during each process,
(c) The efficiency of this cycle, and
(d) The efficiency of a Carnot cycle operating between the temperature extremes of this cycle.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:





