In the production of a bean oil beans containing 13.0 wt% oil and 87.0% solids are ground
Question:
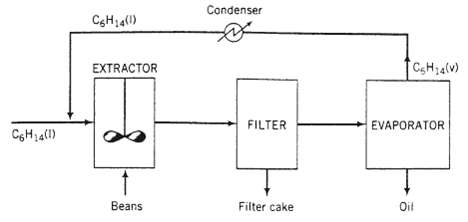
In the production of a bean oil beans containing 13.0 wt% oil and 87.0% solids are ground and fed to a stirred tank (the extractor) along with a recycled stream of liquid n-hexane. The feed ratio is 3kg hexane/kg beans. The ground beans are suspended in the liquid, and essentially all of the oil in the beans is extracted into the hexane. The extractor effluent passes to a filter. The filter cake contains 75.0 wt% bean solids and the balance bean oil and hexane, the latter two in the same ratio in which they emerge from the extractor. The filter cake is discarded and the liquid filtrate is fed to a heated evaporator in which the hexane is vaporized and the oil remains as a liquid. The oil is stored in drums and shipped. The hexane vapor is subsequently cooled and condensed, and the liquid hexane condensate is recycled to the extractor.
(a) Draw and label a flowchart of the process, do the degree-of-freedom analysis, and write in an efficient order the equations you would solve to determine all unknown stream variables, circling the variables for which you would solve.
(b) Calculate the yield of bean oil product (kg oil/kg beans fed), the required fresh hexane feed (kg C6H14/kg beans fed), and the recycle to fresh feed ratio (kg hexane recycled/kg fresh feed).
(c) It has been suggested that a hear exchanger might be added to the process. This process unit would consist of a bundle of parallel metal tubes contained in an outer shell. The liquid filtrate would pass from the filter through the inside of the tubes and then go on to the evaporator. The hot hexane vapor on its way from the evaporator to the extractor would flow through the shell, passing over the outside of the tubes and heating the filtrate. How might the inclusion of this unit lead to a reduction in the operating cost of the process?
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





