A liquid-phase chemical reaction A ? B takes place in a well-stirred tank. The concentration of A
Question:
A liquid-phase chemical reaction A ? B takes place in a well-stirred tank. The concentration of A in the feed is CA0 (mol/m3), and that in the tank and outlet stream is CA (mol/m3). Neither concentration varies with time. The volume of the tank contents is V(m3) and the volumetric flow rate of the inlet and outlet streams is v (m3/s). The reaction rate (the rate at which A is consumed by reaction in the tank) is given by the expression r (mol A consumed/s) = kVCA where k is a constant.
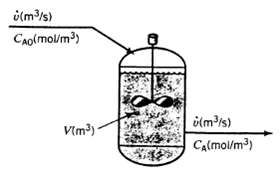
(a) Is this process continuous, batch, or semi batch? Is it transient or steady-state?
(b) What would you expect the reactant concentration CA to equal if k = 0 (no reaction)? What should it approach if k ? ? (infinitely rapid reaction)?
(c) Write a differential balance on A, stating which terms in the general balance equation (accumulation = input + generation ? output ? consumption) you discarded and why you discarded them. Use the balance to derive the following relation between the inlet and outlet reactant concentrations: CA = CA0/1 + kV/v Verity that this relation predicts the results in part (b).
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





