On an uncomfortable summer day, the air is at 87?F and 80% relative humidity. A laboratory air
Question:
On an uncomfortable summer day, the air is at 87?F and 80% relative humidity. A laboratory air conditioner is to deliver 1.00 x 103 ft3/min of air at 55?F in order to maintain the interior air at an average temperature of 75?F and a relative humidity of 40%.
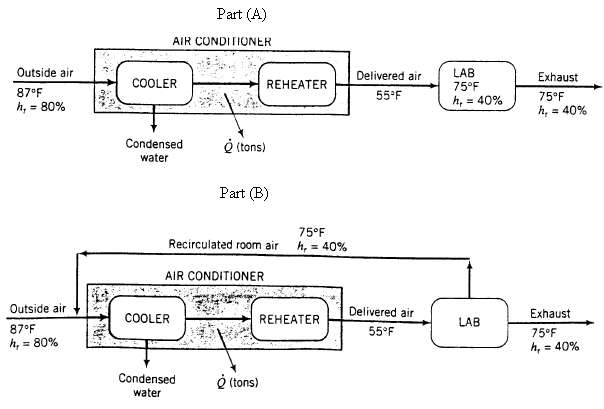
(a) If the vent switch on the air conditioner is turned to the open? position, outside air enters the unit as shown below. In the air conditioner, the air is cooled to a temperature low enough to condense the necessary amount of water and reheated to 55?F, at which point it has the same absolute humidity as the room air. Use the psychometric chart to estimate the rate (lbm/mm) at which water is condensed, the temperature to which the air must be cooled to condense water at this rate, and the net tons of cooling required (Q), where I ton of cooling = ?12,000 Btu/h. [Note: The humid volume of the delivered air (at 55?F). which is difficult to read from the psychometric chart, is 13.07 ft3/lbm dry air, and the heat capacity of liquid water is 1.0 Btu/(lbm ? ?F).]
(b) If the vent switch is set to the ?closed? position (as it normally would be), inside air would be re-circulated through the air conditioner as shown in the following diagram. The recycle ratio (ft3 re-circulated/ft3 exhausted) is 6:1. Calculate the condensation rate and the overall cooling requirement in tons if conditioned air is delivered at the same rate, temperature, and relative humidity as in part (a) what percentage of the cooling load on the air conditioner is saved by re-circulating the air? Explain in your own words why the cooling rate is lower when room air is re-circulated instead of bringing all the air in from the outside.
(c) An even lower cooling load would be required if all of the air passing through the conditioner were re-circulated rather than just 6/7 of it, thereby eliminating the need for outside air and exhaust. Why would this be a bad idea? (Hint: Think about the people working in the laboratory.)
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





