Ozone absorbs ultraviolet radiation in a part of the electromagnetic spectrum energetic enough to disrupt DNA in
Question:
Ozone absorbs ultraviolet radiation in a part of the electromagnetic spectrum energetic enough to disrupt DNA in biological organisms and that is absorbed by no other abundant atmospheric constituent. This spectral range, denoted UV-B, spans the wavelengths of about 290 nm to 320 nm. The molar extinction coefficient of ozone over this range is given in the table below (W.B. DeMore, S.P. Sander, D.M. Golden, R.F. Hampson, M.J. Kurylo, e.J. Howard, A.R. Ravish Ankara, e.E. Kolb, and M.J. Molina, Chemical kinetics and photochemical data for use in stratospheric modeling: Evaluation Number 11, JPL Publication 94-26 (1994).
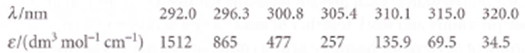
Compute the integrated absorption coefficient of ozone over the wavelength range 290-320 nm. (Hint £ (v) can be fitted to an exponential function quite well.)
Step by Step Answer:






