Phosgene (COCl 2 ) is formed by CO and Cl 2 reacting in the presence of activated
Question:
Phosgene (COCl2) is formed by CO and Cl2 reacting in the presence of activated charcoal: CO + Cl2 ? COCl2?At T = 303.8 K the rate of formation of phosgene in the presence of 1 gram of charcoal is where C denotes concentration in mol/L.
(a) Suppose the charge to a 3.00-liter batch reactor is 1.00 g of charcoal and a gas containing 60 mole% CO and 40 mole% Cl2, and that the initial reactor conditions are 303.8K and 1 atm. Calculate the initial concentrations (mol/L) of both reactants, neglecting the volume occupied by the charcoal. Then, letting Cp (t) be the concentration of phosgene at an arbitrary time r. derive relations for CCO and CCl2 in terms of Cp.
(b) Write a differential balance on phosgene and show that it simplifies to provide an initial condition for this equation.
(c) Starting with the equation of part (b) derive an expression for the time required to achieve a 75% conversion of the limiting reactant. Your solution should have the form t = a definite integral
(d) The integral you derived in part (c) can be evaluated analytically; however, more complex rate laws than the one given for the phosgene formation reaction would yield an integral that must be evaluated numerically. One procedure is to evaluate the integrand at a number of points between the limits of integration and to use a quadrature formula such as the trapezoidal rule or Simpson?s rule (Appendix A.3) to estimate the value of the integral. Use a spreadsheet or write a computer program to evaluate the integrand of the integral of part (c) at n, equally spaced points between and including the limits of integration, where n, is an odd number, and then to evaluate the integral using Simpson's rule. Perform the calculation for np = 5, 21, and 51, and compare the results with the exact value of the integral.
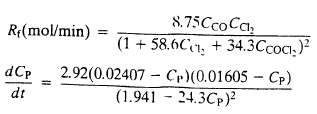
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





