Pressure of an ideal gas. It is desired to get the pressure exerted by an ideal gas
Question:
Pressure of an ideal gas. It is desired to get the pressure exerted by an ideal gas on a wall by accounting for the rate of momentum transfer from the molecules to the wall.?
(a) When a molecule traveling with a velocity v collides with a wall, its incoming velocity components are ux, uy, uz, and after a specular reflection at the wall, its components are ? ux, uy uz. Thus the net momentum transmitted to the wall by a molecule is 2mux. The molecules that have an x- component of the velocity equal to ux, and that will collide with the wall during a small time interval ?t, must be within the volume Sux ?t. How many molecules with velocity components in the range from ux, uy, uz to ux + ?ux, uy + ?uy, uz + ?uz will hit an area S of the wall with a velocity u., within a time interval ?t? It will be f(ux, uy, uz,)dux, duy?duz: times Sux ?t. Then the pressure exerted on the wall by the gas will be Explain carefully how this expression is constructed. Verify that this relation is dimensionally correct.?
(b) Insert Eq. 1C.1-1 for the Maxwell-Boltzmann equilibrium distribution into Eq. 1C.3-1 and perform the integration. Verify that this procedure leads to p = nkT, the ideal gas law. ??
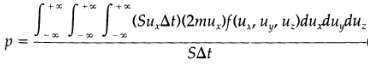
The word "distribution" has several meanings in the financial world, most of them pertaining to the payment of assets from a fund, account, or individual security to an investor or beneficiary. Retirement account distributions are among the most...
Step by Step Answer:






