Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100-105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures
Question:
Shen and Smith [Ind. Eng. Chem. Fundam., 7, 100-105 (1968)] measured equilibrium-adsorption isotherms at four different temperatures for pure benzene vapor on silica gel, having the following properties: surface area = 832 m2/g, pore volume = 0.43 cm3/g, particle density = 1.13 g/cm3, and average pore diameter = 22 A.
The adsorption data are as follows:
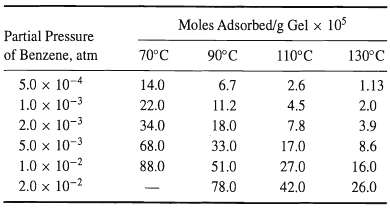
(a) For each temperature, obtain a best fit of the data to (1) linear, (2) Freundlich, and (3) Langmuir isotherms. Which isotherm(s), if any, fit the data reasonably well?
(b) Do the data represent less than a monolayer of adsorption?
(c) From the data, estimate the heat of adsorption. How does this value compare to the heat of vaporization (condensation) ofbenzene?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





