Streams of methane and air (79 mole % N 2 , the balance O 2 ) are
Question:
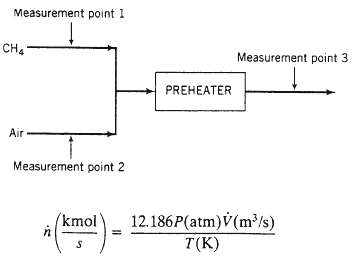
Streams of methane and air (79 mole % N2, the balance O2) are combined at the inlet of a combustion furnace pre-heater. The pressures of each stream are measured with open-end mercury manometers, the temperatures are measured with resistance thermometers, and the volumetric flow rates are measured with orifice meters. Data: ?Flow meter 1: V1 = 947m3/h. Flow meter 2: V2 = 195m3/min. Manometer 1: h1 = 232mm. Manometer 2: h2 = 156mm. Manometer 3: h3 = 74mm. Resistance thermometer 1: r1 = 26.159 ohms. Resistance thermometer 2: r2 = 26.157 ohms. Resistance thermometer 3: r3 = 44.789 ohms. Atmospheric pressure: A sealed-end mercury manometer reads h = 29.76in. The resistance thermometers were calibrated by measuring their resistances at the freezing and boiling points of water, with the following results: T = 0?C: r = 23.624ohms T = 100?C: r = 33.028ohms A straight-line relationship between T and r may be assumed. The relationship between the total molar flow rate of a gas and its volumetric flow rate is, to a good approximation, given by a form of the ideal gas equation of state: where P is the absolute pressure of the gas.
(a) Derive the resistance thermometer calibration formula for T(?C) in terms of r (ohm).
(b) Convert the given gas law expressions to an expression for n(k mol/min) in terms of P(mm Hg), T(?C), and V(m3/min).
(c) Calculate the temperatures and pressures at points 1, 2, and 3.
(d) Calculate the molar flow rate of the combined gas stream.
(e) Calculate the reading of flow meter 3 in m3/min.
(f) Calculate the total mass flow rate and the mass fraction of the methane at point 3.
Step by Step Answer:

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau





