a. Calculate the de Broglie wavelength of the electron in the n = 1, 2, and 3
Question:
a. Calculate the de Broglie wavelength of the electron in the n = 1, 2, and 3 states of the hydrogen atom. Use the information in Table 38.2.
b. Show numerically that the circumference of the orbit for each of these stationary states is exactly equal to n de Broglie wavelengths.
c. Sketch the de Broglie standing wave for the n = 3 orbit.
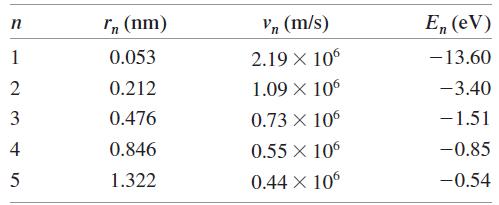
Transcribed Image Text:
In (nm) Vn (m/s) E, (eV) 1 0.053 2.19 X 106 -13.60 2 0.212 1.09 X 106 -3.40 3 0.476 0.73 X 106 -1.51 4 0.846 0.55 X 10 -0.85 1.322 0.44 X 10 -0.54
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
Solve a Using the data in Table 382 the wavelength of the electron in the n 1 state is Lik...View the full answer

Answered By

Sandhya Sharma
I hold M.Sc and M.Phil degrees in mathematics from CCS University, India and also have a MS degree in information management from Asian institute of technology, Bangkok, Thailand. I have worked at a international school in Bangkok as a IT teacher. Presently, I am working from home as a online Math/Statistics tutor. I have more than 10 years of online tutoring experience. My students have always excelled in their studies.
4.90+
119+ Reviews
214+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
Calculate the de Broglie wavelength of a electron in your TV picture tub if it is accelerated by 30,000V. Is it relativistic? How does its wavelength compare to the size of the neck of the tube,...
-
The de Broglie wavelength of an electron in a hydrogen atom is 1.66 nm. Identify the integer n that corresponds to its orbit.
-
Calculate the de Broglie wavelength of an electron moving at 1/137th the speed of light. (At this speed, the relativistic correction to the mass is negligible.)
-
Following procedures similar to those for the homogeneous problem (see Section 8.4.1), develop the following stress field for a pressurized hole in an infinite nonhomogeneous medium with moduli...
-
Suppose GDI' is $1000 billion, the national debt last year was $500 billion, the interest rate paid on government debt is 7%, and GDP is growing by 5% per year. a. If the goal of the government is to...
-
Exchange Limited has an issued Share Capital of 650 7% Redeemable Preference Shares of ~ 100 each and 4,500 Equity Shares of ~ 50 each. The Preference Shares are redeemable at a premium of 10% on...
-
For a derivative of an asset that follows standard geometric Brownian motion, it may be useful to find the sensitivity of the derivative price with respect to a parameter of the underlying process....
-
Assume the following financial data for Rembrandt Paint Co. and Picasso Art Supplies: a. If all the shares of Rembrandt Paint Co. are exchanged for those of Picasso Art Supplies on a share-for-share...
-
How does the implementation of Lean Management methodologies foster organizational agility and enhance operational efficiency in complex business ecosystems ?
-
Consider a class C network with the subnet mask 255.255.255.41. How many network id (NID) and host id (HID) are there in the subnet mask? (A) 24, 3 () 24, 5 () 20, 6 (D) 20, 8
-
Show, by calculation, that the first three states of the hydrogen atom have angular momenta h, 2h, and 3h, respectively.
-
Figure Q 38.12 shows the energy-level diagram of Element X. Figure Q 38.12 a. What is the ionization energy of Element X? b. An atom in the ground state absorbs a photon, then emits a photon with a...
-
Suppose that X1, . . . , Xn form a random sample from the normal distribution with unknown mean and known variance 1, and it is desired to test the following hypotheses: H0: 0.1 0.2, H1: < 0.1...
-
Lowlife Company defaulted on a $ 2 5 0 , 0 0 0 loan that was due on December 3 1 , 2 0 2 1 . The bank has agreed to allow Lowlife to repay the $ 2 5 0 , 0 0 0 by making a series of equal annual...
-
Raphael and Martina are engaged and are planning to travel to Las Vegas during the 2 0 2 2 Christmas season and get married around the end of the year. In 2 0 2 2 , Raphael expects to earn $ 4 5 , 3...
-
Aubrey Beck is single, covered by an employer retirement plan, and she contributed $4,000 to a new Roth IRA on June 16, 2023. On December 29, 2023, she determines that her 2023 modified AGI will...
-
Johan recently sold his 15m boat for an amount of R2 400 000. The original purchase price of the boat was R3 800 000. The boat was not used for trade purposes. Calculate capital gain or capital loss...
-
Nash Company is constructing a building. Construction began on February 1 and was completed on December 3 1 . Expenditures were $ 1 , 8 1 2 , 0 0 0 on March 1 , $ 1 , 2 1 2 , 0 0 0 on June 1 , and $...
-
The S&OP team at Kansas Furniture, has received the following estimates of demand requirements: a) Assuming one-time stockout costs for lost sales of $100 per unit, inventory carrying costs of $25...
-
The slopes of the tangents at the points where the curve y = x2 - 4x intersects the X-axis is 1) 1 2) +2 3) +3 4) +4
-
A piece of modern sculpture consists of an 8.0-m-long, 150 kg stainless steel bar passing diametrically through a 50 kg copper sphere. The center of the sphere is 2.0 m from one end of the bar. To be...
-
Flywheels are large, massive wheels used to store energy. They can be spun up slowly, then the wheels energy can be released quickly to accomplish a task that demands high power. An industrial...
-
Flywheels are large, massive wheels used to store energy. They can be spun up slowly, then the wheels energy can be released quickly to accomplish a task that demands high power. An industrial...
-
Jet Airways borrowed $1,000,000 for one year to improve its liquidity. Jet Airways paid back the principal and interest of $1,060,000 at the end of the year. What is the dollar amount of interest and...
-
if the cost of attending the university is $40,000 per year due at the beginning instead of the end of each year for four years, how much do you have to deposit in a lump sum to be able to pay for...
-
Financial Analysis Case This case can be assigned as a group activity. Additional instructions and material for this activity can be found on the Instructor Resource site and in WileyPLUS. Kenmare...

Study smarter with the SolutionInn App


