a. What downward transitions are possible for a sodium atom in the 6s state? (See Figure 41.24.)
Question:
a. What downward transitions are possible for a sodium atom in the 6s state? (See Figure 41.24.)
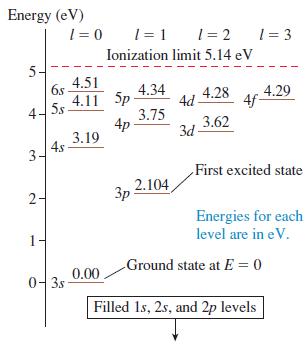
b. What are the wavelengths of the photons emitted in each of these transitions?
Transcribed Image Text:
Energy (eV) 1 = 0 1 = 1 1 = 2 1 = 3 Ionization limit 5.14 eV 5- 4.51 4.34 4.11 5p 3.75 4p 3.19 6s 4.28 4.29 4d 4f- 5s 4 3.62 3d 4s 3- First excited state 2.104 3p Energies for each level are in eV. 1 Ground state at E = 0 0-3s 0.00 Filled Is, 2s, and 2p levels 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Visualize Solve a The allowed transitions are those with l ...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
A sodium atom (Z = 11) contains 11 protons in its nucleus. Strictly speaking, the Bohr model does not apply, because the neutral atom contains 11 electrons instead of a single electron. However, we...
-
What are the wavelengths of the two photons produced when a proton and anti proton at rest annihilate?
-
What are the wavelengths of the two photons produced when a proton and an antiproton at rest annihilate?
-
Discuss the two-pipe system, how it works, and its advantages and disadvantages.
-
A mass of 1.5 kg of air at 120 kPa and 24C is contained in a gas-tight, frictionless piston cylinder device. The air is now compressed to a final pressure of 600 kPa. During the process, heat is...
-
Referring to the lightbulbs described in Question 30 and assuming the emitting areas of the filaments in the two bulbs are the same, which of the two is the brighter? About how much brighter is this...
-
Use the following steps to establish a relationship between the coefficient of determination and the correlation coefficient. a. Show that \(\widehat{y}_{i}-\bar{y}=b_{1}\left(x_{i}-\bar{x} ight)\)....
-
From the perspective of an organization, explain duality as it relates to the give and receive events of an economic exchange.
-
Image transcription text Moist air flow through a duct which contains a heating element. The moist air enters at 95 kPa, 12 C and (I) = 30%; the flow rate is 6 m3/min. Air leaves the heating element...
-
Write a method called average that accepts two integer parameters and returns their average as a floating point value.
-
The 5d 3p transition in the emission spectrum of sodium has a wavelength of 499 nm. What is the energy of the 5d state?
-
Draw a series of pictures, similar to Figure 41.22, for the ground states of Ca, Ni, As, and Kr. 2p 2s %23 %23 1s Z = 5 B 1s25 2p Z = 6 C 1s 2s2p? Z =7 N 1s 2s2p Z = 8 0 1s2s2p* Z = 9 F 1s 2s2p Z =...
-
The pie chart below shows the percent sales of the top five selling fruits in a supermarket. The total sales for one day was 1,000 pounds of these fruits. What is the difference between Peach sales...
-
Question Two. Evaluate the levels of management Question Three. [15 marks] A chief executive officer cannot perform all the activities in organization; discuss the importance of the organizing...
-
QUESTION ONE a) Distinguish between sale and agreement to sell b) Explain the rights of unpaid seller against the goods c) Explain the nature of the contract of hire purchase QUESTION TWO (5 marks)...
-
a) i] Define the term management? [1 marks] ii] Managers must have specific skills and play certain roles in organizations if they are to inspire employees to meet organizational objectives; explain...
-
a) The trial balance of Nyaguthie enterprises prepared as at 30th April, 2016 failed to balance. The difference was posted to a suspense account. On investigation, the following errors were revealed....
-
i. As at 30th June 2013, the following information was available from the records of SGR Limited. ii. Acheque of sh 2,720,000 drawn on 30th June was presented to the bank for payment on 16th July,...
-
Identify how the changes in the internal environment affect the OM strategy for a company. For example, what impact are the following factors likely to have on OM strategy? a. The increased use of...
-
Make an argument that Williams had a right to delay the closing until after August 1.
-
Ann (mass 50 kg) is standing at the left end of a 15-m-long, 500 kg cart that has frictionless wheels and rolls on a frictionless track. Initially both Ann and the cart are at rest. Suddenly, Ann...
-
Force F x = (10 N) sin(2t/4.0 s) is exerted on a 250 g particle during the interval 0s t 2.0s. If the particle starts from rest, what is its speed at t = 2.0 s?
-
Force F x = (10 N) sin(2t/4.0 s) is exerted on a 250 g particle during the interval 0s t 2.0s. If the particle starts from rest, what is its speed at t = 2.0 s?
-
I need to find out how to calculate the 3-year % rate of return. The information I have is a $10,000 investment and a 12-month yield percentage (1.138 for AB company, 1.894 for AIG company, 0.679 for...
-
Anna has an investment that will bring her $100 with a 30% probability and $40 with a 70% probability. Anna's Utility function is U = Y (1/2) . Where Y= income. Anna is considering selling this...
-
on January 1, 2000, the price of koka kola was $10. on jan and, 2020, the shares were worth $100. the stock Paid no dividends during the period. what is the annual geometric return.

Study smarter with the SolutionInn App


