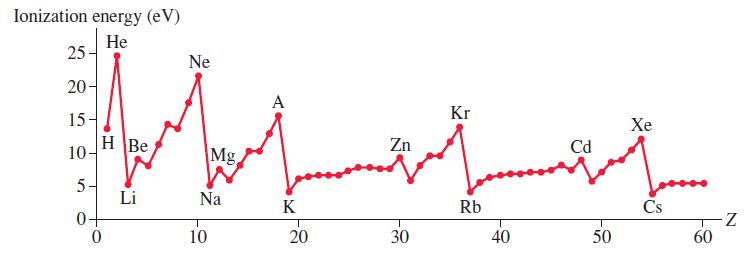
Figure 41.23 shows that the ionization energy of cadmium (Z = 48) is larger than that of
Question:
Figure 41.23 shows that the ionization energy of cadmium (Z = 48) is larger than that of its neighbors. Why is this?
Transcribed Image Text:
Ionization energy (eV) Не 25 Ne 20- A Kr Хе 15 Zn Cd HBe 10- Mg Li Na Rb Cs K z- 60 20 40 50 10 30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 46% (13 reviews)
The groundstate configuration of cadmium Z 48 is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
The ionization energy of a certain element is 412 kJ/mol. When the atoms of this element are in the first excited state, however, the ionization energy is only 126 kJ/mol. Based on this information,...
-
The ionization energy of O2 is smaller than the ionization energy of atomic O; the opposite is true for the ionization energies of N2 and atomic N. Explain this behavior in terms of the molecular...
-
The ionization energy of a certain element is 412 kJ/mol. However, when the atoms of this element are in the first excited state, the ionization energy is only 126 kJ/mol. Based on this information,...
-
Describe what this statement does: print user name = + userName
-
Design an experiment to test if using a mobile device while walking can cause accidents. Be sure that your study controls for threats to internal validity and is ethical.
-
Internal auditors are working to become trusted advisors to management on risk management techniques. Which of the following would be the best way for internal audit to demonstrate they are truly a...
-
Consider the Chapter 2 linear regression model formulas with \(y_{t-1}\) in place of \(x_{t}\), for \(t=2, \ldots, T\). a. Provide an exact expression for \(b_{1}\). b. Provide an exact expression...
-
a. Carlys Catering provides meals for parties and special events. In Chapter 2, you wrote an application that prompts the user for the number of guests attending an event, displays the company motto...
-
Did Keynesian fiscal policy help end the Great Recession of 2007-2009? Explain your answer.
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Predict the ground-state electron configurations of Mg, Sr, and Ba.
-
How many lines of atoms would you expect to see on the collector plate of a Stern-Gerlach apparatus if the experiment is done with (a) Lithium (b) Beryllium? Explain.
-
What happens to the period of a simple pendulum if the pendulums length is doubled? What happens to the period if the mass of the suspended bob is doubled?
-
Tara Palmer Tomkinson and the It Girls Tara Palmer Tomkinson has become one of the most well-known British celebrities over the last ten years. At the age of 20, she was photographed for Tatler...
-
1. What platform would you choose for your restaurant of choice and why? 2. What are the best times to post content for your restaurant of choice? 3. What can you tell about their top two...
-
What are the concepts of the issue regarding students who do not get the most out of their university experience?
-
Recent developments in Indian Psychology and what is its place in the academic arena now?
-
explain The program is designed to equip accounting and finance professionals with the strong critical thinking, problem-solving and technology skills needed to advance business strategy
-
Develop a Process Chart for making a grilled cheese sandwich.
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
The axle in Figure EX12.21 is half the distance from the center to the rim. What is the net torque about the axle? 5.0 kg 30 cm 15 kg |10 kg FIGURE EX12.21
-
The axle in Figure EX12.21 is half the distance from the center to the rim. What is the net torque about the axle? 5.0 kg 30 cm 15 kg |10 kg FIGURE EX12.21
-
A 4.0-m-long, 500 kg steel beam extends horizontally from the point where it has been bolted to the framework of a new building under construction. A 70 kg construction worker stands at the far end...
-
The organisation at which you are employed is keen to use Artificial Intelligence to improve an area of work. This area of work might be related to processes, products, or services. However, your...
-
How do demographic stochasticity, environmental variation, and dispersal dynamics interact to shape the spatial and temporal dynamics of populations within heterogeneous landscapes?
-
A company sells merchandise on November 2 at a $4,000 invoice price with terms of 2/10, n/30. The goods cost $2,000. The company uses the net method to record invoices. The customer pays the balance...

Study smarter with the SolutionInn App


