Figure Q 38.12 shows the energy-level diagram of Element X. Figure Q 38.12 a. What is the
Question:
Figure Q 38.12 shows the energy-level diagram of Element X.
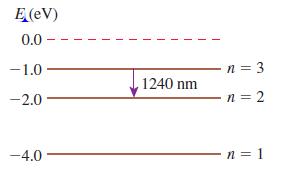
Figure Q 38.12
a. What is the ionization energy of Element X?
b. An atom in the ground state absorbs a photon, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the energy of the photon that was absorbed?
c. An atom in the ground state has a collision with an electron, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the initial kinetic energy of Figure Q 38.5 the electron?
Figure Q 38.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:





