a. Copy and complete the two equations below, which can be used to show the nitration of
Question:
a. Copy and complete the two equations below, which can be used to show the nitration of methylbenzene:
i. C6H5CH3 + NO2+ → — + —
ii. 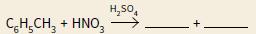
iii. Name the possible mono-substituted products in parts i and ii.
iv. 1-methyl-2,4-dinitrobenzene and 1-methyl- 2,4,6-trinitrobenzene are formed on further nitration of methylbenzene. Draw the displayed formula of each compound.
b. Benzene also undergoes electrophilic substitution when refluxed with fuming sulfuric acid for several hours. This is called sulfonation. The electrophile is the SO3 molecule and the product formed is benzenesulfonic acid, C6H5SO3H.
i Which atom in the SO3 molecule accepts an electron pair in the mechanism of sulfonation?
ii. Write an equation in the style of part a i for the sulfonation of benzene to form benzenesulfonic acid.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





