a. For each of the following changes state whether the entropy of the system decreases or increases.
Question:
a. For each of the following changes state whether the entropy of the system decreases or increases. In each case, explain your answer in terms of the order or disorder of the particles
i. NaCl(s) + aq → Na+(aq) + Cl–(aq)
ii. H2O(g) → H2O(l)
b. The table shows the formula, state and standard molar entropies of the first six straight-chain alkanes.
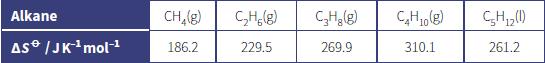
i. Describe and explain the trend in the standard molar entropies of these alkanes.
ii. Estimate the value of the standard molar entropy of the straight-chain alkane with the formula C6H14.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





