a. i. What do you understand by the term relative atomic mass? ii. A sample of boron
Question:
a. i. What do you understand by the term relative atomic mass?
ii. A sample of boron was found to have the following % composition by mass:
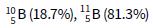
Calculate a value for the relative atomic mass of boron. Give your answer to 3 significant figures.
b. Boron ions, B3+, can be formed by bombarding gaseous boron with high-energy electrons in a mass spectrometer. Deduce the number of electrons in one B3+ ion.
c. Boron is present in compounds called borates.
i. Use the Ar values below to calculate the relative molecular mass of iron(III) borate, Fe(BO2)3. (Ar values: Fe = 55.8, B = 10.8, O = 16.0)
ii. The accurate relative atomic mass of iron, Fe, is 55.8. Explain why the accurate relative atomic mass is not a whole number.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





