a. What is the oxidation number of the transition metal in each of the following complexes? i.
Question:
a. What is the oxidation number of the transition metal in each of the following complexes?
i. [Co(NH3)6]3+
ii. [Ni(CN)4]2–
iii. [Cr(OH)6]3–
iv. [Co(en)3]3+
v. Cu(OH)2(H2O)4
b. EDTA4– ions can act as ligands. A single EDTA4–ion can form six co-ordinate bonds to a central transition metal ion to form an octahedral complex. It is called a hexadentate ligand. Give the formula of such a complex formed between Ni2+and EDTA4–.
c. Which ligands in Table 24.4 are bidentate?
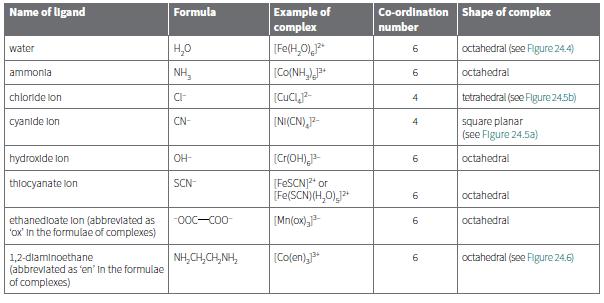
Transcribed Image Text:
Example of complex TFe(H,0)P Name of ligand Formula Co-ordination Shape of complex number water H,0 6. octahedral (see Figure 24.4) ammonia NH, [CO(NH,)J* 6. octahedral chloride lon 4 tetrahedral (see Figure 24.5b) cyanide lon INI(CN),F- CN- square planar (see Figure 24.5a) 4 hydroxide lon OH- (Cr(OH)- octahedral 6. thlocyanate lon (FESCNJ2" or (Fe(SCN)(H,0),P [Mn(ox) SCN octahedral ethanedioate lon (abbrevlated as 'ox' In the formulae of complexes) -O0C-C00- octahedral [Co(en),P* octahedral (see Figure 24.6) 1,2-dlaminoethane (abbrevlated as 'en' in the formulae of complexes) NH,CH,CH,NH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
a Oxidation number of transition metal in the following ...View the full answer

Answered By

Neha Gautam
Dedicated, hard working, creative and energetic teaching style, providing an innovative, stimulating learning environment for students with a solid commitment to their social, academic and behavioral growth and development because I believe every student has potential to rise and shine like a star, they just need guidance and positive attitude towards them. I am resourceful, goal driven and enthusiastic for answering various creative questions with flexibility and adaptability. I want to work for development of student's abilities, skills and knowledge for their growth.
Well said by Nelson Mandela that "EDUCATION IS THE MOST POWERFUL WEAPON WHICH YOU CAN USE TO CHANGE THE WORLD"
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
A complex is written as NiBr2.6NH3 (a) What is the oxidation state of the Ni atom in this complex? (b) What is the likely coordination number for the complex? (c) If the complex is treated with...
-
Find the oxidation numbers of the transition metal in each of the following compounds: a. FeCO3 b. MnO2 c. CuCl2 d. CrO2Cl2
-
Find the oxidation numbers of the transition metal in each of the following compounds: a. CoSO4 b. Ta2O5 c. Cu2(OH)3Cl
-
3 (a) A laser beam of uniform cross-sectional area passes through air into a glass block (refractive index 1.5). Determine the ratios of (i) the velocities (ii) the frequencies and (iii) the...
-
A $1000, 8.5% bond with interest payable annually is purchased six years before maturity to yield 10.5% compounded annually. Compute the premium or discount and the purchase price, and construct the...
-
Astro, Co., recently organized. The company issued no-par common stock to an attorney in exchange for his patent with a market value of $53,000. In addition, Astro, Co., received cash for 4,000...
-
The state of Arizona passed Proposition 200 by popular referendum. This law would require an individual to submit proof of citizenship as a condition of registering to vote. By requiring an...
-
The plant assets section of the comparative balance sheets of Anders Company is reported below. Refer to the balance sheet data above from Anders Company. During 2015, equipment with a book value of...
-
4. 5. In which of following conditions a real gas would behave ideally? (a) Low pressure and low temperature ha (b) At value of temperature equal to its Boyle's temperature (c) Between its critical...
-
Following is the unadjusted trial balance for Alcorn Institute as of December 31, 2011, which initially records prepaid expenses and unearned revenues in balance sheet accounts. The Institute...
-
a. Write two half-equations for the reactions that take place when Fe 2+ (aq) is oxidised by dichromate(VI) ions. b. Combine the two half-equations and write the equation for the oxidation of Fe 2+...
-
Use subshell notation (1s 2 2s 2 2p 6 , etc.) to give the electronic configurations of the following: a. An Fe atom b. A Co 2+ ion c. A Ti 3+ ion.
-
How can the provisions of the SarbanesOxley Act help minimize the likelihood of auditors failing to identify accounting irregularities? Arthur Andersen LLP was founded in Chicago in 1913 by Arthur...
-
Fundamentals of ATC Operation: Search and Rescue (SAR) Procedures how important is the responsibility action on SAR procedures regards to overdue aircraft on or not on the flight plan? And which...
-
There was considerable criticism levied against all levels of government in the aftermath of Hurricane Katrina. In particular, many observers viewed the governmental response as both slow and...
-
According to Scott O'Donnell, Big Apple Circus ' Vice President and General Manager, 70 percent of Big Apple Circus' overall revenues come from the touring unit, with 30 percent coming from donors,...
-
Write a list of variables that revolve around and within your topic of study. Are there variables that are of particular interest or focus in your study? Can you narrow your study down based on your...
-
Alex's Tool and Dye Machine Co If we can't make it, you don't need it! HIGH-LOW METHOD Labor Cost Labor Hours May $ 5,410 585 June $ 4,900 595 July $ 5,570 690 August $ 5,570 680 September $ 5,590...
-
A 6.20-mH inductor is one of the elements in a simple RLC series circuit. When this circuit is connected to a 1.60-kHz sinusoidal source with an rms voltage of 960.0 V, an rms current of 2.50 A lags...
-
An auto-parts manufacturer is considering establishing an engineering computing center. This center will be equipped with three engineering workstations each of which would cost $25,000 and have a...
-
Compound A has molecular formula C 9 H 8 O 2 and exhibits a strong signal at 1740 cm -1 in its IR spectrum. Treatment with two equivalents of LAH followed by water gives the following diol. Identify...
-
Rank each set of compounds in order of increasing acidity: a. b.
-
Malonic acid has two acidic protons: The pKa of the first proton (pK 1 ) is measured to be 2.8, while the pK a of the second proton (pK 2 ) is measured to be 5.7. (a) Explain why the first proton is...
-
Delph Company uses job-order costing with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 55,000 machine-hours would be...
-
The Assembly Department of Interface, Inc., manufacturer of computers, had 500 units of beginning inventory in September, and 4,000 units were transferred to it from the Production Department. The...
-
Shea Furniture started and finished Job 310 during December. The company's records show that the following direct materials were requisitioned for Job 310: (Click the icon to view the direct...

Study smarter with the SolutionInn App


