Compound E has the composition 69.8% carbon, 11.6% hydrogen and 18.6% oxygen. Its mass and 1H NMR
Question:
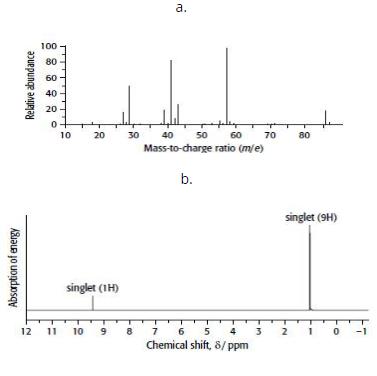
Compound E has the composition 69.8% carbon, 11.6% hydrogen and 18.6% oxygen. Its mass and 1H NMR spectra are shown below.
a. Calculate the empirical formula of E.
b. From the mass spectrum, find the molecular mass of E (ignoring the 13C peak) and hence its molecular formula.
c. Compound E reacts with 2,4-dinitrophenylhydrazine to give a yellow-orange precipitate. Draw displayed formulae for the seven possible isomers of E.
d. Compound E gives a silver mirror with Tollens’ reagent. Identify the functional group in E.
e. Use the 1H NMR spectrum to identify E. Explain your reasoning.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





