The rate of the following reaction between hydrogen peroxide (H 2 O 2 ) and iodide ions
Question:
The rate of the following reaction between hydrogen peroxide (H2O2) and iodide ions can be monitored using sodium thiosulfate and starch indicator:
2H+(aq) + H2O2(aq) + 2I–(aq) → 2H2O(l) + I2(aq)
A mixture of starch solution, potassium iodide solution, sulfuric acid and sodium thiosulfate is made. This mixture can then be reacted with varying concentrations of 10-volume hydrogen peroxide, made by making measured volumes of the peroxide solution up to 25 cm3 with distilled water. When the hydrogen peroxide solution is added to the original mixture containing starch in a flask, the time for the contents of the flask to turn a blue/black colour can be measured. This procedure, using a range of volumes of hydrogen peroxide, can determine how the concentration of hydrogen peroxide affects the rate of the reaction shown above. Here is a set of results obtained from one such investigation.
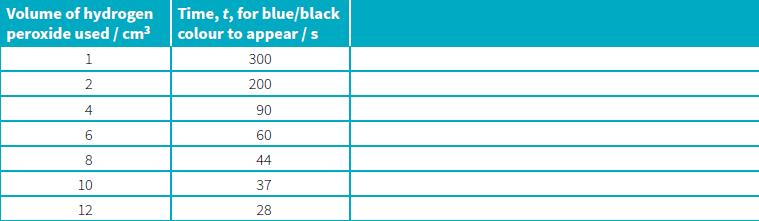
a. A student wants to use these results to draw a graph that will show how the concentration of hydrogen peroxide affects the rate of reaction. Record the heading and values that the student could use to complete the third column of the table (to 2 significant figures).
b. What piece of measuring equipment would be used to make up the volumes of hydrogen peroxide solution to 25 cm3?
c. The student was provided with a stopclock measuring to the nearest second to measure the time taken for the solution to turn blue/black but asked for a stopwatch measuring to one-hundredth of a second. The teacher said that would not be necessary. Explain the teacher’s response.
d. The original mixture was made up using a solution of 40 cm3 of 0.10 mol dm–3 potassium iodide. How many moles of iodide ions are in the reaction mixture?
e. What role does the sodium thiosulfate play in this investigation?
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris




