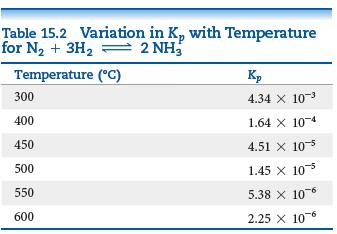
As shown in Table 15.2, the equilibrium constant for the reaction N 2 (g) + 3H 2
Question:
As shown in Table 15.2, the equilibrium constant for the reaction N2(g) + 3H2(g) ⇌ 2 NH3(g) is Kp = 4.34 × 10-3 at 300°C. Pure NH3 is placed in a 1.00-L flask and allowed to reach equilibrium at this temperature. There are 1.05 g NH3 in the equilibrium mixture.
(a) What are the masses of N2 and H2 in the equilibrium mixture?
(b) What was the initial mass of ammonia placed in the vessel?
(c) What is the total pressure in the vessel?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry The Central Science
ISBN: 9780321910417
13th Edition
Authors: Theodore E. Brown, H. Eugene LeMay, Bruce E. Bursten, Catherine Murphy, Patrick Woodward, Matthew E. Stoltzfus
Question Posted:





